Time on Therapy for at Least Three Months Correlates with Overall Survival in Metastatic Renal Cell Carcinoma
- PMID: 31319594
- PMCID: PMC6678132
- DOI: 10.3390/cancers11071000
Time on Therapy for at Least Three Months Correlates with Overall Survival in Metastatic Renal Cell Carcinoma
Abstract
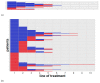
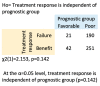
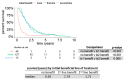
With 15 drugs currently approved for the treatment of metastatic renal cell carcinoma (mRCC) and even more combination regimens with immunotherapy on the horizon, there remains a distinct lack of molecular biomarkers for therapeutic efficacy. Our study reports on real-world clinical outcomes of mRCC patients from a tertiary academic medical center treated with empirically selected standard-of-care therapy. We utilized the Stanford Renal Cell Carcinoma Database (RCCD) to report on various outcome measures, including overall survival (OS) and the median number of lines of targeted therapies received from the time of metastatic diagnosis. We found that most metastatic patients did not survive long enough to attempt even half of the available targeted therapies. We also noted that patients who failed to receive a clinical benefit within the first two lines of therapy could still go on to experience clinical benefit in later lines of therapy. The term, "clinical benefit" was assigned to a line of therapy if a patient remained on drug treatment for three months or longer. Moreover, patients with clinical benefit in at least one line of therapy experienced significantly longer OS compared to those who did not have clinical benefit in at least one line of therapy. Developing biomarkers that identify patients who will receive clinical benefit in individual lines of therapy is one potential strategy for achieving rational drug sequencing in mRCC.
Keywords: IMDC criteria; RCC; favorable-, poor-, and intermediate-risk prognosis RCC; immunotherapy; kidney cancer; metastatic renal cell carcinoma; systemic treatment; targeted kinase inhibitors; therapy sequencing.
Conflict of interest statement
A.C.F. is the founder of Molecular Decisions, Inc. The other authors declare no conflict of interest.
Figures



References
-
- American Cancer Society | Cancer Facts & Statistics. [(accessed on 26 January 2019)]; Available online: https://cancerstatisticscenter.cancer.org.
-
- Kidney and Renal Pelvis Statistics American Cancer Society. [(accessed on 31 October 2018)]; Available online: https://cancerstatisticscenter.cancer.org/#!/cancer-site/Kidney%20and%20....
-
- Heng D.Y., Mackenzie M.J., Vaishampayan U.N., Bjarnason G.A., Knox J.J., Tan M.H., Wood L., Wang Y., Kollmannsberger C., North S., et al. Primary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: Clinical characteristics, risk factors, and subsequent therapy. Ann. Oncol. 2012;23:1549–1555. doi: 10.1093/annonc/mdr533. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

