DNA supercoiling and transcription in bacteria: a two-way street
- PMID: 31319794
- PMCID: PMC6639932
- DOI: 10.1186/s12860-019-0211-6
DNA supercoiling and transcription in bacteria: a two-way street
Abstract
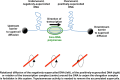
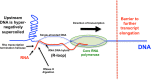
Background: The processes of DNA supercoiling and transcription are interdependent because the movement of a transcription elongation complex simultaneously induces under- and overwinding of the DNA duplex and because the initiation, elongation and termination steps of transcription are all sensitive to the topological state of the DNA.
Results: Policing of the local and global supercoiling of DNA by topoisomerases helps to sustain the major DNA-based transactions by eliminating barriers to the movement of transcription complexes and replisomes. Recent data from whole-genome and single-molecule studies have provided new insights into how interactions between transcription and the supercoiling of DNA influence the architecture of the chromosome and how they create cell-to-cell diversity at the level of gene expression through transcription bursting.
Conclusions: These insights into fundamental molecular processes reveal mechanisms by which bacteria can prevail in unpredictable and often hostile environments by becoming unpredictable themselves.
Conflict of interest statement
The author confirms that he has no competing interests.
Figures

References
-
- Bauer WR, Crick FHC, White JH. Supercoiled DNA. Sci Am 1980;243(1):100–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

