Correlation of anti-acetylcholine receptor antibody levels and long-term outcomes of juvenile myasthenia gravis in Taiwan: a case control study
- PMID: 31319795
- PMCID: PMC6637626
- DOI: 10.1186/s12883-019-1397-0
Correlation of anti-acetylcholine receptor antibody levels and long-term outcomes of juvenile myasthenia gravis in Taiwan: a case control study
Abstract
Background: Myasthenia gravis is the most common disease affecting the neuromuscular junction. The most common etiology among patients with juvenile myasthenia gravis is the production of antibodies against the acetylcholine receptor. However, the clinical outcome in relation to serum levels of anti-acetylcholine receptor antibodies in juvenile myasthenia gravis has rarely been discussed. We aimed to analyze the correlation between the presence of anti-acetylcholine receptor antibodies and outcome in juvenile myasthenia gravis.
Methods: Patients diagnosed with juvenile myasthenia gravis younger than of 20 years of age were retrospectively recruited from January 1995 to February 2017 in a tertiary referral medical center. According to the Myasthenia Gravis Foundation of America outcome scale, the primary outcome was complete symptom remission and cessation of medications for at least 1 year measured 2 years after diagnosis. Secondary outcome was complete symptom remission at the last outpatient clinic.
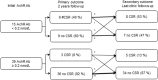
Results: A total of 54 patients were followed up for over 2 years. Nine patients (9/54, 16.7%) achieved complete remission without medication use at 2 years after diagnosis. Thirteen (24.1%) patients achieved complete remission during longer follow-up periods. Those with negative anti-acetylcholine receptor antibodies were more likely to achieve complete remission at 2 years (6/15 [40%] vs. 3/39 [7.7%], 95% Confidence interval [CI] 1.670 to 38.323) and at the last outpatient clinic follow-up (8/15 [53.3%] vs. 5/39 [12.8%], 95% CI 2.367 to 20.704). Thirteen patients with comorbid autoimmune thyroid diseases were older than those without disease (11.8 ± 5.8 years old vs. 8.0 ± 6.3 years old, 95% CI 0.018 to 7.33). Moreover, patients negative for anti-acetylcholine receptor antibodies were less likely comorbid with autoimmune thyroid disease (1/35 [2.9%] vs. 12/71 [16.9%], 95% CI 0.018 to 1.161).
Conclusions: Juvenile myasthenia gravis patients without anti-acetylcholine antibodies exhibited significantly increased complete remission rates and a reduced likelihood of comorbid autoimmune thyroid diseases compared with those with anti-acetylcholine receptor antibodies among Chinese.
Keywords: Acetylcholine receptors; Asia; Graves’ disease; Juvenile; Myasthenia gravis; Outcome assessment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

