HBV-related hepatocarcinogenesis: the role of signalling pathways and innovative ex vivo research models
- PMID: 31319796
- PMCID: PMC6637598
- DOI: 10.1186/s12885-019-5916-6
HBV-related hepatocarcinogenesis: the role of signalling pathways and innovative ex vivo research models
Abstract
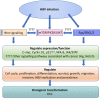
Background: Hepatitis B virus (HBV) is the leading cause of liver cancer, but the mechanisms by which HBV causes liver cancer are poorly understood and chemotherapeutic strategies to cure liver cancer are not available. A better understanding of how HBV requisitions cellular components in the liver will identify novel therapeutic targets for HBV associated hepatocellular carcinoma (HCC).
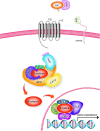
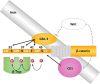
Main body: The development of HCC involves deregulation in several cellular signalling pathways including Wnt/FZD/β-catenin, PI3K/Akt/mTOR, IRS1/IGF, and Ras/Raf/MAPK. HBV is known to dysregulate several hepatocyte pathways and cell cycle regulation resulting in HCC development. A number of these HBV induced changes are also mediated through the Wnt/FZD/β-catenin pathway. The lack of a suitable human liver model for the study of HBV has hampered research into understanding pathogenesis of HBV. Primary human hepatocytes provide one option; however, these cells are prone to losing their hepatic functionality and their ability to support HBV replication. Another approach involves induced-pluripotent stem (iPS) cell-derived hepatocytes. However, iPS technology relies on retroviruses or lentiviruses for effective gene delivery and pose the risk of activating a range of oncogenes. Liver organoids developed from patient-derived liver tissues provide a significant advance in HCC research. Liver organoids retain the characteristics of their original tissue, undergo unlimited expansion, can be differentiated into mature hepatocytes and are susceptible to natural infection with HBV.
Conclusion: By utilizing new ex vivo techniques like liver organoids it will become possible to develop improved and personalized therapeutic approaches that will improve HCC outcomes and potentially lead to a cure for HBV.
Keywords: Cell cycle; Hepatitis B virus; Liver cancer; Organoids; Wnt signalling.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

