Repeat adverse drug events associated with outpatient medications: a descriptive analysis of 3 observational studies in British Columbia, Canada
- PMID: 31320328
- PMCID: PMC6639095
- DOI: 10.9778/cmajo.20180190
Repeat adverse drug events associated with outpatient medications: a descriptive analysis of 3 observational studies in British Columbia, Canada
Abstract
Background: Adverse drug events are an important cause of preventable emergency department visits and hospital admissions. We examined repeat adverse drug events associated with outpatient medications resulting in acute care utilization.
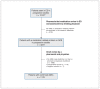
Methods: This descriptive analysis combined data from 3 prospective multicentre observational studies, in which clinical pharmacists and physicians independently evaluated patients who visited the emergency department for adverse drug events in 3 hospitals in British Columbia. During these studies, an independent committee adjudicated all discordant and uncertain cases using a standardized algorithm. For the current study, we retrospectively reviewed the medical and research records of all patients 19 years of age and older who had been diagnosed with an adverse drug event during the primary studies to determine the proportion of repeat events. We used multivariable logistic regression to identify factors associated with repeat events; we adjusted for clustering at the hospital level for patient-level analyses and at the patient level for event-level analyses.
Results: Among 12 977 patients, 1178 were diagnosed with 1296 adverse drug events at the point of care. Of these events, 32.5% (421 of 1296; 95% confidence interval [CI] 29.8%-35.1%) were repeat events, of which 75.3% (317 of 421; 95% CI 71.1%-79.5%) were deemed probably or definitely preventable as re-exposure to the culprit medication or repeat withdrawal of an indicated medication was inconsistent with best medical practice. Patients presenting with repeat events were more likely to have renal failure (odds ratio [OR] 2.01; 95% CI 1.32%-3.07%) or a mental health diagnosis (OR 1.39; 95% CI 1.02%-1.88%).
Interpretation: A high proportion of adverse drug events were repeat events, most of which were deemed preventable. Interventions to ensure that care providers are aware of previously diagnosed adverse drug events when prescribing or dispensing need to be developed and evaluated and may reduce unintentional re-exposures to previously harmful medications.
Copyright 2019, Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: The authors’ research group is developing a software application called ActionADE, which will enable standardized documentation of adverse drug events by front-line clinicians and communication of this information to a central medication dispensing database. No other competing interests were declared.
Figures
References
-
- Budnitz DS, Lovegrove MC, Shehab N, et al. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–12. - PubMed
-
- Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666–71. - PubMed
-
- Research Priority Setting Working Group. Global priorities for research in patient safety. 1st ed. Geneva: World Health Organization; 2008.
-
- Medication without harm: WHO Global Patient Safety Challenge. Geneva: World Health Organization; 2017.
LinkOut - more resources
Full Text Sources