BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle
- PMID: 31320589
- PMCID: PMC6690026
- DOI: 10.1073/pnas.1900544116
BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle
Abstract
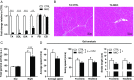
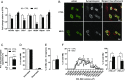
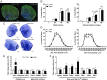
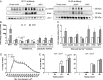
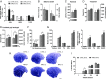
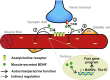
Brain-derived neurotrophic factor (BDNF) influences the differentiation, plasticity, and survival of central neurons and likewise, affects the development of the neuromuscular system. Besides its neuronal origin, BDNF is also a member of the myokine family. However, the role of skeletal muscle-derived BDNF in regulating neuromuscular physiology in vivo remains unclear. Using gain- and loss-of-function animal models, we show that muscle-specific ablation of BDNF shifts the proportion of muscle fibers from type IIB to IIX, concomitant with elevated slow muscle-type gene expression. Furthermore, BDNF deletion reduces motor end plate volume without affecting neuromuscular junction (NMJ) integrity. These morphological changes are associated with slow muscle function and a greater resistance to contraction-induced fatigue. Conversely, BDNF overexpression promotes a fast muscle-type gene program and elevates glycolytic fiber number. These findings indicate that BDNF is required for fiber-type specification and provide insights into its potential modulation as a therapeutic target in muscle diseases.
Keywords: endurance exercise; myokine; neuromuscular junction; neurotrophic factor; oxidative fiber.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chao M. V., Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 4, 299–309 (2003). - PubMed
-
- Huang E. J., Reichardt L. F., Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 (2003). - PubMed
-
- Chevrel G., Hohlfeld R., Sendtner M., The role of neurotrophins in muscle under physiological and pathological conditions. Muscle Nerve 33, 462–476 (2006). - PubMed
-
- Kablar B., Rudnicki M. A., Development in the absence of skeletal muscle results in the sequential ablation of motor neurons from the spinal cord to the brain. Dev. Biol. 208, 93–109 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

