Increase in HDAC9 suppresses myoblast differentiation via epigenetic regulation of autophagy in hypoxia
- PMID: 31320610
- PMCID: PMC6639330
- DOI: 10.1038/s41419-019-1763-2
Increase in HDAC9 suppresses myoblast differentiation via epigenetic regulation of autophagy in hypoxia
Abstract
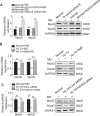
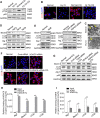
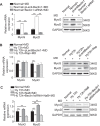
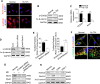
Extremely reduced oxygen (O2) levels are detrimental to myogenic differentiation and multinucleated myotube formation, and chronic exposure to high-altitude hypoxia has been reported to be an important factor in skeletal muscle atrophy. However, how chronic hypoxia causes muscle dysfunction remains unknown. In the present study, we found that severe hypoxia (1% O2) significantly inhibited the function of C2C12 cells (from a myoblast cell line). Importantly, the impairment was continuously manifested even during culture under normoxic conditions for several passages. Mechanistically, we revealed that histone deacetylases 9 (HDAC9), a member of the histone deacetylase family, was significantly increased in C2C12 cells under hypoxic conditions, thereby inhibiting intracellular autophagy levels by directly binding to the promoter regions of Atg7, Beclin1, and LC3. This phenomenon resulted in the sequential dephosphorylation of GSK3β and inactivation of the canonical Wnt pathway, impairing the function of the C2C12 cells. Taken together, our results suggest that hypoxia-induced myoblast dysfunction is due to aberrant epigenetic regulation of autophagy, and our experimental evidence reveals the possible molecular pathogenesis responsible for some muscle diseases caused by chronic hypoxia and suggests a potential therapeutic option.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


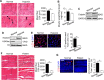
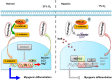
Similar articles
-
The Autophagy Regulatory Molecule CSRP3 Interacts with LC3 and Protects Against Muscular Dystrophy.Int J Mol Sci. 2020 Jan 23;21(3):749. doi: 10.3390/ijms21030749. Int J Mol Sci. 2020. PMID: 31979369 Free PMC article.
-
Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation.Autophagy. 2019 Sep;15(9):1606-1619. doi: 10.1080/15548627.2019.1591672. Epub 2019 Apr 7. Autophagy. 2019. PMID: 30859901 Free PMC article.
-
TRAF6 inhibits colorectal cancer metastasis through regulating selective autophagic CTNNB1/β-catenin degradation and is targeted for GSK3B/GSK3β-mediated phosphorylation and degradation.Autophagy. 2019 Sep;15(9):1506-1522. doi: 10.1080/15548627.2019.1586250. Epub 2019 Mar 4. Autophagy. 2019. PMID: 30806153 Free PMC article.
-
HDAC9 inhibition reduces skeletal muscle atrophy and enhances regeneration in mice with cigarette smoke-induced COPD.Biochim Biophys Acta Mol Basis Dis. 2024 Mar;1870(3):167023. doi: 10.1016/j.bbadis.2024.167023. Epub 2024 Jan 11. Biochim Biophys Acta Mol Basis Dis. 2024. PMID: 38218381
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
Hypoxic Signaling in Skeletal Muscle Maintenance and Regeneration: A Systematic Review.Front Physiol. 2021 Jun 23;12:684899. doi: 10.3389/fphys.2021.684899. eCollection 2021. Front Physiol. 2021. PMID: 34248671 Free PMC article.
-
Role of Histone Deacetylases in Skeletal Muscle Physiology and Systemic Energy Homeostasis: Implications for Metabolic Diseases and Therapy.Front Physiol. 2020 Aug 11;11:949. doi: 10.3389/fphys.2020.00949. eCollection 2020. Front Physiol. 2020. PMID: 32848876 Free PMC article. Review.
-
IGF-1 Signaling Regulates Mitochondrial Remodeling during Myogenic Differentiation.Nutrients. 2022 Mar 16;14(6):1249. doi: 10.3390/nu14061249. Nutrients. 2022. PMID: 35334906 Free PMC article.
-
Synergistic Effects of Multiple Factors Involved in COVID-19-dependent Muscle Loss.Aging Dis. 2022 Apr 1;13(2):344-352. doi: 10.14336/AD.2021.0817. eCollection 2022 Apr. Aging Dis. 2022. PMID: 35371610 Free PMC article.
-
Quis Custodiet Ipsos Custodes (Who Controls the Controllers)? Two Decades of Studies on HDAC9.Life (Basel). 2021 Jan 27;11(2):90. doi: 10.3390/life11020090. Life (Basel). 2021. PMID: 33513699 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

