Metabolomic Analysis of Skeletal Muscle in Aged Mice
- PMID: 31320689
- PMCID: PMC6639307
- DOI: 10.1038/s41598-019-46929-8
Metabolomic Analysis of Skeletal Muscle in Aged Mice
Abstract
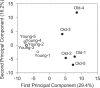
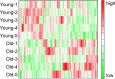
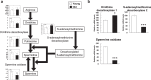
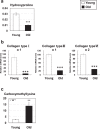
Sarcopenia is the age-induced, progressive loss of skeletal muscle mass and function. To better understand changes in skeletal muscle during sarcopenia, we performed a metabolomic analysis of skeletal muscle in young (8-week-old) and aged (28-month-old) mice by using capillary electrophoresis with electrospray ionization time-of-flight mass spectrometry. Principal component analysis showed clear changes in metabolites between young and aged mice. Glucose metabolism products were decreased in aged mice, specifically fructose 1,6-diphosphate (0.4-fold) and dihydroxyacetone phosphate (0.6-fold), possibly from decreased glycolytic muscle fibers. Multiple metabolic products associated with phospholipid metabolism were significantly changed in aged mice, which may reflect changes in cell membrane phospholipids of skeletal muscle. Products of polyamine metabolism, which are known to increase nucleic acid and protein synthesis, decreased in spermine (0.5-fold) and spermidine (0.6-fold) levels. By contrast, neurotransmitter levels were increased in skeletal muscle of aged mice, including acetylcholine (1.8-fold), histamine (2.6-fold), and serotonin (1.7-fold). The increase in acetylcholine might compensate for age-associated dropout of neuromuscular junctions, whereas the increases in histamine and serotonin might be due to muscle injury associated with aging. Further analysis focusing on the altered metabolites observed in this study will provide essential data for understanding aging muscles.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

