Treatment Strategies Based on Histological Targets against Invasive and Resistant Glioblastoma
- PMID: 31320900
- PMCID: PMC6610731
- DOI: 10.1155/2019/2964783
Treatment Strategies Based on Histological Targets against Invasive and Resistant Glioblastoma
Abstract
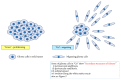
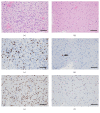
Glioblastoma (GBM) is the most common and the most malignant primary brain tumor and is characterized by rapid proliferation, invasion into surrounding normal brain tissues, and consequent aberrant vascularization. In these characteristics of GBM, invasive properties are responsible for its recurrence after various therapies. The histomorphological patterns of glioma cell invasion have often been referred to as the "secondary structures of Scherer." The "secondary structures of Scherer" can be classified mainly into four histological types as (i) perineuronal satellitosis, (ii) perivascular satellitosis, (iii) subpial spread, and (iv) invasion along the white matter tracts. In order to develop therapeutic interventions to mitigate glioma cell migration, it is important to understand the biological mechanism underlying the formation of these secondary structures. The main focus of this review is to examine new molecular pathways based on the histopathological evidence of GBM invasion as major prognostic factors for the high recurrence rate for GBMs. The histopathology-based pharmacological and biological targets for treatment strategies may improve the management of invasive and resistant GBMs.
Figures



Similar articles
-
Satellitosis, a Crosstalk between Neurons, Vascular Structures and Neoplastic Cells in Brain Tumours; Early Manifestation of Invasive Behaviour.Cancers (Basel). 2020 Dec 11;12(12):3720. doi: 10.3390/cancers12123720. Cancers (Basel). 2020. PMID: 33322379 Free PMC article. Review.
-
Hans Joachim Scherer and His Impact on the Diagnostic, Clinical, and Modern Research Aspects of Glial Tumors.Cureus. 2019 Nov 13;11(11):e6148. doi: 10.7759/cureus.6148. Cureus. 2019. PMID: 31886082 Free PMC article. Review.
-
Rac1+ cells distributed in accordance with CD 133+ cells in glioblastomas and the elevated invasiveness of CD 133+ glioma cells with higher Rac1 activity.Chin Med J (Engl). 2012 Dec;125(24):4344-8. Chin Med J (Engl). 2012. PMID: 23253699
-
Live-Cell Imaging Assays to Study Glioblastoma Brain Tumor Stem Cell Migration and Invasion.J Vis Exp. 2018 Aug 29;(138):58152. doi: 10.3791/58152. J Vis Exp. 2018. PMID: 30222149 Free PMC article.
-
ACTC1 as an invasion and prognosis marker in glioma.J Neurosurg. 2017 Feb;126(2):467-475. doi: 10.3171/2016.1.JNS152075. Epub 2016 Apr 15. J Neurosurg. 2017. PMID: 27081897
Cited by
-
Recognition of a Novel Gene Signature for Human Glioblastoma.Int J Mol Sci. 2022 Apr 9;23(8):4157. doi: 10.3390/ijms23084157. Int J Mol Sci. 2022. PMID: 35456975 Free PMC article.
-
Biomimetic Approach of Brain Vasculature Rapidly Characterizes Inter- and Intra-Patient Migratory Diversity of Glioblastoma.Small Methods. 2024 Dec;8(12):e2400210. doi: 10.1002/smtd.202400210. Epub 2024 May 15. Small Methods. 2024. PMID: 38747088 Free PMC article.
-
Brain and Breast Cancer Cells with PTEN Loss of Function Reveal Enhanced Durotaxis and RHOB Dependent Amoeboid Migration Utilizing 3D Scaffolds and Aligned Microfiber Tracts.Cancers (Basel). 2021 Oct 14;13(20):5144. doi: 10.3390/cancers13205144. Cancers (Basel). 2021. PMID: 34680293 Free PMC article.
-
Megalencephalic leukoencephalopathy with subcortical cysts 1 (MLC1) promotes glioblastoma cell invasion in the brain microenvironment.Oncogene. 2020 Dec;39(50):7253-7264. doi: 10.1038/s41388-020-01503-9. Epub 2020 Oct 10. Oncogene. 2020. PMID: 33040087 Free PMC article.
-
Artificial intelligence-based locoregional markers of brain peritumoral microenvironment.Sci Rep. 2023 Jan 18;13(1):963. doi: 10.1038/s41598-022-26448-9. Sci Rep. 2023. PMID: 36653382 Free PMC article.
References
-
- Scherer H. J. Structural development in gliomas. American Journal of Cancer. 1938;34(3):333–351.
Publication types
LinkOut - more resources
Full Text Sources

