The Protective Effect of Sonneratia apetala Fruit Extract on Acetaminophen-Induced Liver Injury in Mice
- PMID: 31320915
- PMCID: PMC6607706
- DOI: 10.1155/2019/6919834
The Protective Effect of Sonneratia apetala Fruit Extract on Acetaminophen-Induced Liver Injury in Mice
Abstract
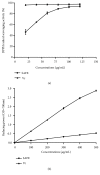
Acute liver injury is a common consequence of taking overdose of acetaminophen (APAP). The aim of this study was to evaluate the antioxidant activity and hepatoprotective effect of a mangrove plant Sonneratia apetala fruit extract (SAFE) on APAP-induced liver injury in mice. Mice were orally pretreated with SAFE (100, 200, and 400 mg/kg) daily for one week. The control and APAP groups were intragastrically administered with distilled water, and NAC group was treated with N-Acetyl-L-cysteine (NAC) before APAP exposure. The results manifested that SAFE significantly improved survival rates, attenuated hepatic histological damage, and decreased the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in serum in APAP-exposed mice. SAFE treatment also increased glutathione (GSH) level and glutathione peroxidase (GSH-Px) activity, enhanced catalase (CAT), and total antioxidant capacity (T-AOC), as well as reducing malondialdehyde (MDA) level in liver. In addition, the formation of tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and elevation of myeloperoxidase (MPO) in APAP-exposed mice were inhibited after SAFE treatment. And SAFE also displayed high DPPH radical scavenging activity and reducing power in vitro. The main bioactive components of SAFE such as total phenol, flavonoid, condensed tannin, and carbohydrate were determined. The current study proved that SAFE exerted potential protective effect against APAP-induced acute liver injury, which might be associated with the antioxidant and anti-inflammatory activities of SAFE.
Figures






References
-
- McGill M. R., Sharpe M. R., Williams C. D., Taha M., Curry S. C., Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. The Journal of Clinical Investigation. 2012;122(4):1574–1583. doi: 10.1172/JCI59755. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

