Stay Fit, Stay Young: Mitochondria in Movement: The Role of Exercise in the New Mitochondrial Paradigm
- PMID: 31320983
- PMCID: PMC6607712
- DOI: 10.1155/2019/7058350
Stay Fit, Stay Young: Mitochondria in Movement: The Role of Exercise in the New Mitochondrial Paradigm
Erratum in
-
Corrigendum to "Stay Fit, Stay Young: Mitochondria in Movement: The Role of Exercise in the New Mitochondrial Paradigm".Oxid Med Cell Longev. 2021 Jan 18;2021:9274841. doi: 10.1155/2021/9274841. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33574988 Free PMC article.
Abstract
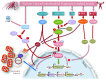
Skeletal muscles require the proper production and distribution of energy to sustain their work. To ensure this requirement is met, mitochondria form large networks within skeletal muscle cells, and during exercise, they can enhance their functions. In the present review, we discuss recent findings on exercise-induced mitochondrial adaptations. We emphasize the importance of mitochondrial biogenesis, morphological changes, and increases in respiratory supercomplex formation as mechanisms triggered by exercise that may increase the function of skeletal muscles. Finally, we highlight the possible effects of nutraceutical compounds on mitochondrial performance during exercise and outline the use of exercise as a therapeutic tool in noncommunicable disease prevention. The resulting picture shows that the modulation of mitochondrial activity by exercise is not only fundamental for physical performance but also a key point for whole-organism well-being.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

