Development and Characterization of a Dry Powder Formulation for Anti-Tuberculosis Drug Spectinamide 1599
- PMID: 31321552
- PMCID: PMC7851762
- DOI: 10.1007/s11095-019-2666-8
Development and Characterization of a Dry Powder Formulation for Anti-Tuberculosis Drug Spectinamide 1599
Abstract
Purpose: Human tuberculosis (TB) is a global health problem that causes nearly 2 million deaths per year. Anti-TB therapy exists, but it needs to be administered as a cocktail of antibiotics for six months. This lengthy therapy results in low patient compliance and is the main reason attributable to the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains of Mycobacterium tuberculosis.
Methods: One alternative approach is to combine anti-TB multidrug therapy with inhalational TB therapy. The aim of this work was to develop and characterize dry powder formulations of spectinamide 1599 and ensure in vitro and in vivo delivered dose reproducibility using custom dosators.
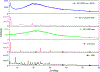
Results: Amorphous dry powders of spectinamide 1599 were successfully spray dried with mass median aerodynamic diameter (MMAD) = 2.32 ± 0.05 μm. The addition of L-leucine resulted in minor changes to the MMAD (1.69 ± 0.35 μm) but significantly improved the inhalable portion of spectinamide 1599 while maintaining amorphous qualities. Additionally, we were able to demonstrate reproducibility of dry powder administration in vitro and in vivo in mice.
Conclusions: The corresponding systemic drug exposure data indicates dose-dependent exposure in vivo in mice after dry powder intrapulmonary aerosol delivery in the dose range 15.4 - 32.8 mg/kg.
Keywords: aerosol; inhalation; spectinamide; tuberculosis.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Development of a proliposomal pretomanid dry powder inhaler as a novel alternative approach for combating pulmonary tuberculosis.Int J Pharm. 2024 Oct 25;664:124608. doi: 10.1016/j.ijpharm.2024.124608. Epub 2024 Aug 18. Int J Pharm. 2024. PMID: 39163929
-
Inhalable spray-dried formulation of D-LAK antimicrobial peptides targeting tuberculosis.Int J Pharm. 2015 Aug 1;491(1-2):367-74. doi: 10.1016/j.ijpharm.2015.07.001. Epub 2015 Jul 4. Int J Pharm. 2015. PMID: 26151107
-
Preclinical Evaluation of Inhalational Spectinamide-1599 Therapy against Tuberculosis.ACS Infect Dis. 2021 Oct 8;7(10):2850-2863. doi: 10.1021/acsinfecdis.1c00213. Epub 2021 Sep 21. ACS Infect Dis. 2021. PMID: 34546724 Free PMC article.
-
High dose dry powder inhalers to overcome the challenges of tuberculosis treatment.Int J Pharm. 2018 Oct 25;550(1-2):398-417. doi: 10.1016/j.ijpharm.2018.08.061. Epub 2018 Sep 1. Int J Pharm. 2018. PMID: 30179703 Review.
-
Dry powder inhalable formulations for anti-tubercular therapy.Adv Drug Deliv Rev. 2016 Jul 1;102:83-101. doi: 10.1016/j.addr.2016.05.011. Epub 2016 May 17. Adv Drug Deliv Rev. 2016. PMID: 27212477 Review.
Cited by
-
The YAP/SERCA2a signaling pathway protects cardiomyocytes against reperfusion-induced apoptosis.Aging (Albany NY). 2020 Jul 9;12(13):13618-13632. doi: 10.18632/aging.103481. Epub 2020 Jul 9. Aging (Albany NY). 2020. PMID: 32645692 Free PMC article.
-
Excipients for Novel Inhaled Dosage Forms: An Overview.AAPS PharmSciTech. 2024 Feb 14;25(2):36. doi: 10.1208/s12249-024-02741-w. AAPS PharmSciTech. 2024. PMID: 38356031 Review.
-
Spray dried tigecycline dry powder aerosols for the treatment of Nontuberculous mycobacterial pulmonary infections.Tuberculosis (Edinb). 2023 Mar;139:102306. doi: 10.1016/j.tube.2023.102306. Epub 2023 Jan 20. Tuberculosis (Edinb). 2023. PMID: 36716525 Free PMC article.
-
A modified BPaL regimen for tuberculosis treatment replaces linezolid with inhaled spectinamides.Elife. 2024 Oct 8;13:RP96190. doi: 10.7554/eLife.96190. Elife. 2024. PMID: 39378165 Free PMC article.
-
Pharmacokinetic Considerations for Optimizing Inhaled Spray-Dried Pyrazinoic Acid Formulations.Mol Pharm. 2023 Sep 4;20(9):4491-4504. doi: 10.1021/acs.molpharmaceut.3c00199. Epub 2023 Aug 17. Mol Pharm. 2023. PMID: 37590399 Free PMC article.
References
-
- WorldHealthOrganization. Tuberculosis Key Facts. WHO Press; Available from: http://www.who.int/en/news-room/fact-sheets/detail/tuberculosis.
-
- Murray S, Mendel C, Spigelman M. TB Alliance regimen development for multidrug-resistant tuberculosis. The International Journal of Tuberculosis and Lung Disease. 2016;20(12):S38-S41. - PubMed
-
- Misra A, Hickey AJ, Rossi C, Borchard G, Terada H, Makino K, Fourie PB, Colombo P. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis. 2011;91(1):71–81. - PubMed
-
- Hickey AJ, Durham PG, Dharmadhikari A, Nardell EA. Inhaled drug treatment for tuberculosis: Past progress and future prospects. Journal of Controlled Release. 2016;240:127–134. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

