The efficacy of drug-eluting beads bronchial arterial chemoembolization loaded with gemcitabine for treatment of non-small cell lung cancer
- PMID: 31321919
- PMCID: PMC6718028
- DOI: 10.1111/1759-7714.13139
The efficacy of drug-eluting beads bronchial arterial chemoembolization loaded with gemcitabine for treatment of non-small cell lung cancer
Abstract
Background: Drug-eluting beads bronchial arterial chemoembolization (DEB-BACE) can embolize the tumor-feeding artery and also be loaded with antitumor drugs, which can be released slowly into the local tumor environment. The effect of DEB-BACE in patients with lung cancer remains unclear. We evaluated the efficacy and safety of DEB-BACE with gemcitabine-loaded CalliSpheres beads in patients with non-small cell lung cancer (NSCLC).
Methods: From May 2017 to December 2018, six patients with NSCLC who were ineligible or refused to receive standard treatment underwent DEB-BACE with gemcitabine-loaded CalliSpheres beads. The primary endpoint was the objective response rate (ORR). The secondary endpoints were progression-free survival (PFS), overall survival (OS), and quality of life. Safety was evaluated by the occurrences of adverse events and serious adverse events.
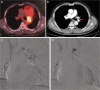
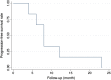
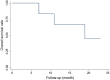
Results: All patients were treated with DEB-BACE loaded with gemcitabine (800 mg) using CalliSpheres beads. Five patients also received transarterial infusion with nedaplatin (80-100 mg). Of the six patients, five underwent a second session of DEB-BACE, with intervals of one month between the first and second session. The median follow-up time was 16.5 months (7.0-23.0 months). ORR and disease control rate were 50.0% and 100.0%, 50.0% and 83.3%, 50.0% and 66.7% respectively at 2, 4, and 6 months after DEB-BACE. One patient maintained a partial response and the other five had progressive disease, of whom two patients died and the other three remained alive receiving targeted therapy, radiotherapy, transarterial infusion or thermal ablation. The median PFS was 8.0 months (4-23 months), and the 6- and 12 month PFS rates were 66.7% and 16.7%, respectively. The median OS was 16.5 months (7-23 months), and the six and 12 month OS rates were 100.0% and 66.7%, respectively. Hemoptysis, cough and dyspnea disappeared after DEB-BACE in four patients. Global quality of life, physical and emotional functioning were all significantly improved at two months (P < 0.05). There were no serious adverse events.
Conclusions: DEB-BACE with gemcitabine-loaded CalliSpheres beads is a feasible and well-tolerated treatment for patients with NSCLC who are ineligible or refuse to receive standard treatment.
Keywords: Bronchial arterial chemoembolization; CalliSpheres® beads; gemcitabine; non-small cell lung cancer.
© 2019 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures
Similar articles
-
Bevacizumab loaded CalliSpheres® bronchial arterial chemoembolization combined with immunotherapy and targeted therapy for advanced lung adenocarcinoma.Front Pharmacol. 2023 May 22;14:1170344. doi: 10.3389/fphar.2023.1170344. eCollection 2023. Front Pharmacol. 2023. PMID: 37284322 Free PMC article.
-
Bronchial arterial chemoembolization with Drug-Eluting beads plus sequential chemotherapy for the treatment of stage III and IV lung squamous cell carcinoma.Eur J Radiol. 2024 Jun;175:111398. doi: 10.1016/j.ejrad.2024.111398. Epub 2024 Mar 1. Eur J Radiol. 2024. PMID: 38579540
-
Bronchial artery chemoembolization with drug-eluting beads versus bronchial artery infusion followed by polyvinyl alcohol particles embolization for advanced squamous cell lung cancer: A retrospective study.Eur J Radiol. 2023 Apr;161:110747. doi: 10.1016/j.ejrad.2023.110747. Epub 2023 Feb 16. Eur J Radiol. 2023. PMID: 36821958
-
Super-selective transcatheter vesical arterial chemoembolization with drug-loaded beads for muscle-invasive bladder cancer with hematuria.Abdom Radiol (NY). 2023 Feb;48(2):780-786. doi: 10.1007/s00261-022-03748-2. Epub 2022 Dec 7. Abdom Radiol (NY). 2023. PMID: 36477632 Review.
-
Improving quality of life in patients with non-small cell lung cancer: research experience with gemcitabine.Eur J Cancer. 1997 Jan;33 Suppl 1:S8-13. doi: 10.1016/s0959-8049(96)00336-x. Eur J Cancer. 1997. PMID: 9166093 Review.
Cited by
-
One-step fabrication of lidocaine/CalliSpheres® composites for painless transcatheter arterial embolization.J Transl Med. 2022 Oct 11;20(1):463. doi: 10.1186/s12967-022-03653-8. J Transl Med. 2022. PMID: 36221084 Free PMC article.
-
Clinical outcomes of gemcitabine-loaded callispheres drug-eluting beads for patients with advanced and inoperable lung cancer: A case series study.Front Pharmacol. 2022 Sep 29;13:992526. doi: 10.3389/fphar.2022.992526. eCollection 2022. Front Pharmacol. 2022. PMID: 36249775 Free PMC article.
-
Transcatheter drug delivery through bronchial artery for COVID-19: is it fiction or could it come true?Eur Radiol Exp. 2020 Jul 6;4(1):42. doi: 10.1186/s41747-020-00171-4. Eur Radiol Exp. 2020. PMID: 32632766 Free PMC article. Review.
-
Drug-eluting beads-transcatheter arterial chemoembolization with or without iodine-125 treatment is effective and tolerable in treating advanced non-small cell lung cancer patients: a pilot study.Transl Cancer Res. 2020 May;9(5):3191-3202. doi: 10.21037/tcr.2020.03.64. Transl Cancer Res. 2020. PMID: 35117685 Free PMC article.
-
Efficacy and Safety of Drug-Eluting Bead Bronchial Arterial Chemoembolization Plus Anlotinib in Patients With Advanced Non-small-Cell Lung Cancer.Front Cell Dev Biol. 2021 Oct 29;9:768943. doi: 10.3389/fcell.2021.768943. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34778275 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. - PubMed
-
- Reck M, Popat S, Reinmuth N et al Metastatic non‐small‐cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014; 25 (Suppl 3): iii27–39. - PubMed
-
- Gridelli C, Rossi A, Carbone DP e a. Non‐small‐cell lung cancer. Nat Rev Dis Primers 2015; 1: 15009. - PubMed
-
- Du L, Morgensztern D. Chemotherapy for advanced‐stage non‐small cell lung cancer. Cancer J 2015; 21: 366–70. - PubMed
-
- Kwan KC. Oral bioavailability and first‐pass effects. Drug Metab Dispos 1997; 25: 1329–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

