Design and synthesis of phthalazine-based compounds as potent anticancer agents with potential antiangiogenic activity via VEGFR-2 inhibition
- PMID: 31322015
- PMCID: PMC6691788
- DOI: 10.1080/14756366.2019.1642883
Design and synthesis of phthalazine-based compounds as potent anticancer agents with potential antiangiogenic activity via VEGFR-2 inhibition
Abstract
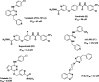
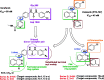
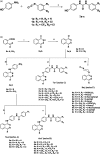
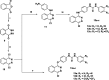
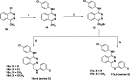
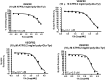
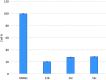
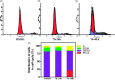
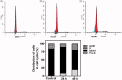
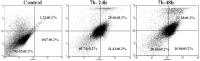
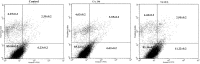
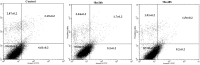
In the designed compounds, either a biarylamide or biarylurea moiety or an N-substituted piperazine motif was linked to position 1 of the phthalazine core. The anti-proliferative activity of the synthesised compounds revealed that eight compounds (6b, 6e, 7b, 13a, 13c, 16a, 16d and 17a) exhibited excellent broad spectrum cytotoxic activity in NCI 5-log dose assays against the full 60 cell panel with GI50 values ranging from 0.15 to 8.41 µM. Moreover, the enzymatic assessment of the synthesised compounds against VEGFR-2 tyrosine kinase showed the significant inhibitory activities of the biarylureas (12b, 12c and 13c) with IC50s of 4.4, 2.7 and 2.5 μM, respectively, and with 79.83, 72.58 and 71.6% inhibition of HUVEC at 10 μM, respectively. Additionally, compounds (7b, 13c and 16a) were found to induce cell cycle arrest at S phase boundary. Compound 7b triggered a concurrent increase in cleaved caspase-3 expression level, indicating the apoptotic-induced cell death.
Keywords: Substituted phthalazines; VEGFR-2 kinase inhibitors; anti-proliferative; apoptosis.
Figures



Similar articles
-
Design, Synthesis, In Vitro Anti-cancer Activity, ADMET Profile and Molecular Docking of Novel Triazolo[3,4-a]phthalazine Derivatives Targeting VEGFR-2 Enzyme.Anticancer Agents Med Chem. 2018;18(8):1184-1196. doi: 10.2174/1871520618666180412123833. Anticancer Agents Med Chem. 2018. PMID: 29651967
-
N-Substituted-4-phenylphthalazin-1-amine-derived VEGFR-2 inhibitors: Design, synthesis, molecular docking, and anticancer evaluation studies.Arch Pharm (Weinheim). 2021 Mar;354(3):e2000219. doi: 10.1002/ardp.202000219. Epub 2020 Nov 16. Arch Pharm (Weinheim). 2021. PMID: 33197080
-
Increasing the binding affinity of VEGFR-2 inhibitors by extending their hydrophobic interaction with the active site: Design, synthesis and biological evaluation of 1-substituted-4-(4-methoxybenzyl)phthalazine derivatives.Eur J Med Chem. 2016 May 4;113:50-62. doi: 10.1016/j.ejmech.2016.02.029. Epub 2016 Feb 15. Eur J Med Chem. 2016. PMID: 26922228
-
Phthalazinone Scaffold: Emerging Tool in the Development of Target Based Novel Anticancer Agents.Anticancer Agents Med Chem. 2020;20(18):2228-2245. doi: 10.2174/1871520620666200807220146. Anticancer Agents Med Chem. 2020. PMID: 32767957 Review.
-
Synthesis and biological activity of structurally diverse phthalazine derivatives: A systematic review.Bioorg Med Chem. 2019 Sep 15;27(18):3979-3997. doi: 10.1016/j.bmc.2019.07.050. Epub 2019 Aug 1. Bioorg Med Chem. 2019. PMID: 31401008
Cited by
-
Rationale, in silico docking, ADMET profile, design, synthesis and cytotoxicity evaluations of phthalazine derivatives as VEGFR-2 inhibitors and apoptosis inducers.RSC Adv. 2024 Aug 27;14(37):27110-27121. doi: 10.1039/d4ra04956j. eCollection 2024 Aug 22. RSC Adv. 2024. PMID: 39193307 Free PMC article.
-
Synthesis of novel phthalazine-based derivatives with potent cytotoxicity against HCT-116 cells through apoptosis and VEGFR2 inhibition.RSC Adv. 2024 Apr 24;14(19):13027-13043. doi: 10.1039/d4ra02103g. eCollection 2024 Apr 22. RSC Adv. 2024. PMID: 38660526 Free PMC article.
-
A green microwave method for synthesizing a more stable phthalazin-1-ol isomer as a good anticancer reagent using chemical plasma organic reactions.Heliyon. 2021 Mar 8;7(3):e06220. doi: 10.1016/j.heliyon.2021.e06220. eCollection 2021 Mar. Heliyon. 2021. PMID: 33748447 Free PMC article.
-
Synthesis of phthalazine-based derivatives as selective anti-breast cancer agents through EGFR-mediated apoptosis: in vitro and in silico studies.BMC Chem. 2023 Jul 27;17(1):90. doi: 10.1186/s13065-023-00995-2. BMC Chem. 2023. PMID: 37501139 Free PMC article.
-
Repurposing Study of 4-Acyl-1-phenylaminocarbonyl-2-substituted-piperazine Derivatives as Potential Anticancer Agents-In Vitro Evaluation against Breast Cancer Cells.Int J Mol Sci. 2023 Dec 1;24(23):17041. doi: 10.3390/ijms242317041. Int J Mol Sci. 2023. PMID: 38069364 Free PMC article.
References
-
- Zhang YS, Liu Y, Chen D, et al. . Synthesis and antitumor activities of novel 1,4-disubstituted phthalazine derivatives. Eur. J. Med. Chem 2010;45:3504–10. - PubMed
-
- Imramovský A, Jorda R, Pauk K, et al. . Substituted 2-hydroxy-N-(arylalkyl)benzamides induce apoptosis in cancer cell lines. Eur J Med Chem 2013;68:253–9. - PubMed
-
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 2009;9:28–39. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
