Molecular mechanism of Forkhead box M1 inhibition by thiostrepton in breast cancer cells
- PMID: 31322278
- PMCID: PMC6667886
- DOI: 10.3892/or.2019.7225
Molecular mechanism of Forkhead box M1 inhibition by thiostrepton in breast cancer cells
Abstract
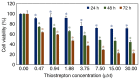
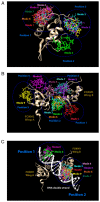
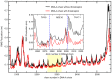
Breast cancer is the most common type of malignancies in women worldwide, and genotoxic chemotherapeutic drugs are effective by causing DNA damage in cancer cells. However, >90% of patients with metastatic cancer are resistant to chemotherapy. The Forkhead box M1 (FOXM1) transcription factor plays a pivotal role in the resistance of breast cancer cells to chemotherapy by promoting DNA damage repair following genotoxic drug treatment. The aim of the present study was to investigate the inhibition of the FOXM1 protein by thiostrepton, a natural antibiotic produced by the Streptomyces species. Experimental studies were designed to examine the effectiveness of thiostrepton in downregulating FOXM1 mRNA expression and activity, leading to senescence and apoptosis of breast cancer cells. The cytotoxicity of thiostrepton in breast cancer was determined using cell viability assay. Additionally, thiostrepton treatment decreased the mRNA expression of cyclin B1 (CCNB1), a downstream target of FOXM1. The present results indicated that thiostrepton inhibited FOXM1 mRNA expression and its effect on CCNB1. Molecular dynamic simulations were performed to study the interactions between FOXM1‑DNA and thiostrepton after molecular docking. The results revealed that the possible mechanism underlying the inhibitory effect of thiostrepton on FOXM1 function was by forming a tight complex with the DNA and FOXM1 via its binding domain. Collectively, these results indicated that thiostrepton is a specific and direct inhibitor of the FOXM1 protein in breast cancer. The findings of the present study may lead to the development of novel therapeutic strategies for breast cancer and help overcome resistance to conventional chemotherapeutic drugs.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

