Impact of circulating tumor DNA mutant allele fraction on prognosis in RAS-mutant metastatic colorectal cancer
- PMID: 31322322
- PMCID: PMC6717744
- DOI: 10.1002/1878-0261.12547
Impact of circulating tumor DNA mutant allele fraction on prognosis in RAS-mutant metastatic colorectal cancer
Abstract
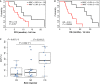
Despite major advances in the treatment of metastatic colorectal cancer (mCRC), the survival rate remains very poor. This study aims at exploring the prognostic value of RAS-mutant allele fraction (MAF) in plasma in mCRC. Forty-seven plasma samples from 37 RAS-mutated patients with nonresectable metastases were tested for RAS in circulating tumor DNA using BEAMing before first- and/or second-line treatment. RAS MAF was correlated with several clinical parameters (number of metastatic sites, hepatic volume, carcinoembryonic antigen, CA19-9 levels, primary site location, and treatment line) and clinical outcome [progression-free survival (PFS) and overall survival (OS)]. An independent cohort of 32 patients from the CAPRI-GOIM trial was assessed for clinical outcome based on plasma baseline MAF. RAS MAF analysis at baseline revealed a significant correlation with longer OS [Hazard ratios (HR) = 3.514; P = 0.00066]. Patients with lower MAF also showed a tendency to longer PFS, although not statistically significant. Multivariate analysis showed RAS MAFs as an independent prognostic factor in both OS (HR = 2.73; P = 0.006) and first-line PFS (HR = 3.74; P = 0.049). Tumor response to treatment in patients with higher MAF was progression disease (P = 0.007). Patients with low MAFs at baseline in the CAPRI-GOIM group also showed better OS [HR = 3.84; 95% confidence intervals (CI) 1.5-9.6; P = 0.004] and better PFS (HR = 2.5; 95% CI: 1.07-5.62; P = 0.033). This minimally invasive test may help in adding an independent factor to better estimate outcomes before initiating treatment. Further prospective studies using MAF as a stratification factor could further validate its utility in clinical practice.
Keywords: MAF; RAS analysis; circulating tumor DNA; metastatic colorectal cancer; prognostic biomarker.
© 2019 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
JT has served in a consulting or advisory role for Amgen, Boehringer Ingelheim, Celgene, Chugai, ImClone, Lilly, Merck, S.L., Madrid, Merck Serono, Millennium Pharmaceuticals, Inc., Novartis, Roche, Sanofi, and Taiho. RS has served in a consulting or advisory role for Amgen, Merck, S.L., Madrid, and Roche Dx and obtained research funding from Roche Dx. AV has served in a consulting or advisory role for Merck, S.L., Madrid, Merck Serono, and Sysmex. All remaining authors have declared no conflicts of interest.
Figures


References
-
- Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J et al (2017) Prognostic and predictive value of primary tumour side in patients with RAS wild‐type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 28, 1713–1729. - PMC - PubMed
-
- Azuara D, Santos C, Lopez‐Doriga A, Grasselli J, Nadal M, Sanjuan X, Marin F, Vidal J, Montal R, Moreno V et al (2016) Nanofluidic digital PCR and extended genotyping of RAS and BRAF for improved selection of metastatic colorectal cancer patients for Anti‐EGFR therapies. Mol Cancer Ther 15, 1106–1112. - PubMed
-
- Ciardiello F, Normanno N, Maiello E, Martinelli E, Troiani T, Pisconti S, Giuliani F, Barone C, Cartenì G, Rachiglio AM et al (2014) Clinical activity of FOLFIRI plus cetuximab according to extended gene mutation status by next‐generation sequencing: findings from the CAPRI‐GOIM trial. Ann Oncol 25, 1756–1761. - PubMed
-
- Contal C and O'Quigley J (1999) An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal 30, 253–270.

