Approaching Clinical Trials in Childhood Interstitial Lung Disease and Pediatric Pulmonary Fibrosis
- PMID: 31322415
- PMCID: PMC6945798
- DOI: 10.1164/rccm.201903-0544CI
Approaching Clinical Trials in Childhood Interstitial Lung Disease and Pediatric Pulmonary Fibrosis
Abstract
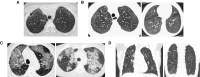
Childhood interstitial lung disease (chILD) comprises a spectrum of rare diffuse lung disorders. chILD is heterogeneous in origin, with different disease manifestations occurring in the context of ongoing lung development. The large number of disorders in chILD, in combination with the rarity of each diagnosis, has hampered scientific and clinical progress within the field. Epidemiologic and natural history data are limited. The prognosis varies depending on the etiology, with some forms progressing to lung transplant or death. There are limited treatment options for patients with chILD. Although U.S. Food and Drug Administration-approved treatments are now available for adult patients with idiopathic pulmonary fibrosis, no clinical trials have been conducted in a pediatric population using agents designed to treat lung fibrosis. This review will focus on progressive chILD disorders and on the urgent need for meaningful objective outcome measures to define, detect, and monitor fibrosis in children.
Keywords: child; interstitial; lung diseases; rheumatologic; surfactant.
Figures

References
-
- Deterding RR. Children’s interstitial and diffuse lung disease: progress and future horizons. Ann Am Thorac Soc. 2015;12:1451–1457. - PubMed
-
- Kurland G, Deterding RR, Hagood JS, Young LR, Brody AS, Castile RG, et al. American Thoracic Society Committee on Childhood Interstitial Lung Disease (chILD) and the chILD Research Network. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188:376–394. - PMC - PubMed
-
- Fan LL, Dishop MK, Galambos C, Askin FB, White FV, Langston C, et al. Children’s Interstitial and Diffuse Lung Disease Research Network (chILDRN) Diffuse lung disease in biopsied children 2 to 18 years of age: application of the chILD classification scheme. Ann Am Thorac Soc. 2015;12:1498–1505. - PMC - PubMed
-
- Dinwiddie R, Sharief N, Crawford O. Idiopathic interstitial pneumonitis in children: a national survey in the United Kingdom and Ireland. Pediatr Pulmonol. 2002;34:23–29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

