Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond
- PMID: 31322645
- PMCID: PMC7341904
- DOI: 10.1210/er.2019-00007
Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond
Abstract
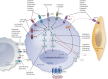
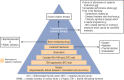
The treatment of advanced thyroid cancer has undergone rapid evolution in the last decade, with multiple kinase inhibitor drug approvals for each subtype of thyroid cancer and a number of other commercially available drugs that have been studied for this indication. Although most of the US Food and Drug Administration (FDA)-approved drugs are antiangiogenic multikinase inhibitors-vandetanib, cabozantinib, sorafenib, lenvatinib-there are two FDA indications that are mutation specific-dabrafenib/trametinib for BRAF-mutated anaplastic thyroid cancer and larotrectinib for NTRK-fusion thyroid cancer. Furthermore, other mutation-specific drugs, immunotherapies, and novel strategies for advanced thyroid cancer are under investigation. Understanding the molecular basis of thyroid cancer, the drugs of interest for treatment of advanced thyroid cancer, and how these drugs can be administered safely and in the appropriate clinical scenario are the topics of this review.
Copyright © 2019 Endocrine Society.
Figures
References
-
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. - PubMed
-
- Simpson WJ, McKinney SE, Carruthers JS, Gospodarowicz MK, Sutcliffe SB, Panzarella T. Papillary and follicular thyroid cancer. Prognostic factors in 1,578 patients. Am J Med. 1987;83(3):479–488. - PubMed
-
- Gopal RK, Kubler K, Calvo SE, Polak P, Livitz D, Rosebrock D, Sadow PM, Campbell B, Donovan SE, Amin S, Gigliotti BJ, Grabarek Z, Hess JM, Stewart C, Braunstein LZ, Arndt PF, Mordecai S, Shih AR, Chaves F, Zhan T, Lubitz CC, Kim J, Iafrate AJ, Wirth L, Parangi S, Leshchiner I, Daniels GH, Mootha VK, Dias-Santagata D, Getz G, McFadden DG. Widespread chromosomal losses and mitochondrial DNA alterations as genetic drivers in hurthle cell carcinoma. Cancer Cell. 2018;34(2):242–255.e5. - PMC - PubMed
-
- Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, Nanjangud G, Eng S, Bose P, Kuo F, Morris LGT, Landa I, Carrillo Albornoz PB, Riaz N, Nikiforov YE, Patel K, Umbricht C, Zeiger M, Kebebew E, Sherman E, Ghossein R, Fagin JA, Chan TA. Integrated genomic analysis of hurthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell. 2018;34(2):256–270.e5. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

