Microcosting Analysis of Diffuse Large B-Cell Lymphoma Treatment in Malawi
- PMID: 31322992
- PMCID: PMC6690619
- DOI: 10.1200/JGO.19.00059
Microcosting Analysis of Diffuse Large B-Cell Lymphoma Treatment in Malawi
Abstract
Purpose: To describe the cost of treating diffuse large B-cell lymphoma (DLBCL) in Malawi under the following circumstances: (1) palliation only, (2) first-line cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), (3) salvage etoposide, ifosfamide, and cisplatin (EPIC), and (4) salvage gemcitabine and oxaliplatin (GEMOX).
Methods: We conducted a microcosting analysis from the health system perspective in the context of a prospective cohort study at a national teaching hospital in Lilongwe, Malawi. Clinical outcomes data were derived from previously published literature from the cohort. Cost data were collected for treatment and 2-year follow-up, reflecting costs incurred by the research institution or referral hospital for goods and services. Costs were collected in Malawian kwacha, inflated and converted to 2017 US dollars.
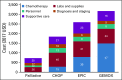
Results: On a per-patient basis, palliative care alone cost $728 per person. Total costs for first-line treatment with CHOP chemotherapy was $1,844, of which chemotherapy drugs made up 15%. Separate salvage EPIC and GEMOX cost $2,597 and $3,176, respectively. Chemotherapy drugs accounted for 30% of EPIC and 47% of GEMOX.
Conclusion: To our knowledge, this is among the first published efforts to characterize detailed costs of cancer treatment in sub-Saharan Africa. The per-patient cost of first-line treatment of DLBCL in Malawi is low relative to high-income countries, suggesting that investments in fixed-duration, curative-intent DLBCL treatment may be attractive in sub-Saharan Africa. Salvage treatment of relapsed/refractory DLBCL costs much more than first-line therapy. Formal cost-effectiveness modeling for CHOP and salvage treatment in the Malawian and other low-resource settings is needed to inform decision makers about optimal use of resources for cancer treatment.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
No potential conflicts of interest were reported.
Figures
References
-
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Basu A.Estimating costs and valuations of non-health benefits in cost-effectiveness analysis Neumann PJ, Ganiats TG, Russell LB, et al.Cost-Effectiveness in Health and Medicine Ed 2Oxford, United Kingdom: Oxford University Press; 2016, p 22
-
- Wood L, Robinson R, Gavine L, et al. A single unit lymphoma experience: Outcome in a Cape Town academic centre. Transfus Apheresis Sci. 2007;37:93–102. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

