Novel protective and risk loci in hip dysplasia in German Shepherds
- PMID: 31323019
- PMCID: PMC6668854
- DOI: 10.1371/journal.pgen.1008197
Novel protective and risk loci in hip dysplasia in German Shepherds
Abstract
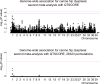
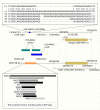
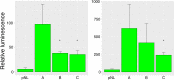
Canine hip dysplasia is a common, non-congenital, complex and hereditary disorder. It can inflict severe pain via secondary osteoarthritis and lead to euthanasia. An analogous disorder exists in humans. The genetic background of hip dysplasia in both species has remained ambiguous despite rigorous studies. We aimed to investigate the genetic causes of this disorder in one of the high-risk breeds, the German Shepherd. We performed genetic analyses with carefully phenotyped case-control cohorts comprising 525 German Shepherds. In our genome-wide association studies we identified four suggestive loci on chromosomes 1 and 9. Targeted resequencing of the two loci on chromosome 9 from 24 affected and 24 control German Shepherds revealed deletions of variable sizes in a putative enhancer element of the NOG gene. NOG encodes for noggin, a well-described bone morphogenetic protein inhibitor affecting multiple developmental processes, including joint development. The deletion was associated with the healthy controls and mildly dysplastic dogs suggesting a protective role against canine hip dysplasia. Two enhancer variants displayed a decreased activity in a dual luciferase reporter assay. Our study identifies novel loci and candidate genes for canine hip dysplasia, with potential regulatory variants in the NOG gene. Further research is warranted to elucidate how the identified variants affect the expression of noggin in canine hips, and what the potential effects of the other identified loci are.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: HL provides consultancy to Genoscoper Laboratories Oy, a canine DNA diagnostics company, he used to partially own during the study (till 12/2017). There are no other competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

