Interactome Changes Quantified to Identify the ER Proteostasis Network to Fight Amyloid Diseases
- PMID: 31323219
- PMCID: PMC7102898
- DOI: 10.1016/j.chembiol.2019.07.003
Interactome Changes Quantified to Identify the ER Proteostasis Network to Fight Amyloid Diseases
Abstract
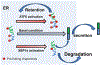
In this issue of Cell Chemical Biology, Plate et al. (2019) used quantitative interactome proteomics to define the molecular mechanism by which ATF6 activation reduces amyloidogenic protein secretion. These results shed light on preventing the amyloid formation at the very early step to treat devastating amyloid diseases.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
Comment on
-
Quantitative Interactome Proteomics Reveals a Molecular Basis for ATF6-Dependent Regulation of a Destabilized Amyloidogenic Protein.Cell Chem Biol. 2019 Jul 18;26(7):913-925.e4. doi: 10.1016/j.chembiol.2019.04.001. Epub 2019 May 16. Cell Chem Biol. 2019. PMID: 31105062 Free PMC article.
References
-
- Balch WE, Morimoto RI, Dillin A, and Kelly JW (2008). Adapting proteostasis for disease intervention. Science 319, 916–919. - PubMed
-
- Chiti F, and Dobson CM (2017). Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu. Rev. Biochem 86, 27–68. - PubMed
-
- Hetz C, and Papa FR (2018). The unfolded protein response and cell fate control. Mol. Cell 69, 169–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

