Noninvasive characterization of in situ forming implant diffusivity using diffusion-weighted MRI
- PMID: 31323243
- PMCID: PMC6815723
- DOI: 10.1016/j.jconrel.2019.07.019
Noninvasive characterization of in situ forming implant diffusivity using diffusion-weighted MRI
Abstract
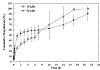
In situ forming implants (ISFIs) form a solid drug-eluting depot, releasing drug for an extended period of time after a minimally-invasive injection. Clinical use of ISFIs has been limited because many factors affect drug release kinetics. The aim of this study was to use diffusion-weighted MRI (DWI) to noninvasively quantify spatial-temporal changes in implant diffusivity in situ. ISFIs were formed using poly(lactic-co-glycolic) acid, with a molecular weight of either 15 kDa or 52 kDa, and fluorescein as the mock drug. Drug release, polymer erosion, polymer degradation, and implant diffusivity were analyzed in vitro over 21 days. DWI was also performed in vivo over 5 days. Spatial diffusivity maps of the implant were generated using DWI data. Results showed constant diffusivity at the implant shell ((1.17 ± 0.13) × 10-3 mm2/s) and increasing diffusivity within the interior over time (from (0.268 ± 0.081) × 10-3 mm2/s during day 1 to (1.88 ± 0.04) × 10-3 mm2/s at 14 d), which correlated with increasing porosity of the implant microstructure. Implants formed in vivo followed the same diffusivity trend as those in vitro. This study validates the use of DWI to provide novel functional information about implant behavior through its ability to noninvasively characterize transport properties within the implant both in vitro and in vivo.
Keywords: Controlled release; Diffusion-weighted imaging; Diffusivity; Drug delivery; MRI; in situ forming implants.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declarations of interest: none
Figures





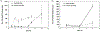


References
-
- Wertheimer A, Santella T, Finestone A, Levy R. Clinical and Economic Advantages of Modern Dosage Forms: Improving Medication Adherence [Internet]. Npcnow.org. 2006. [cited 2018 Nov 4]. Available from: https://www.npcnow.org/system/files/research/download/Clinical-and-Econo...
-
- Dunn R, English J, Cowsar D, Vanderbilt D. Biodegradable in-situ forming implants and methods of producing the same. USA; US4938763A, 1988.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

