Durability of Spontaneous and Treatment-Related Loss of Hepatitis B s Antigen
- PMID: 31323381
- PMCID: PMC6962568
- DOI: 10.1016/j.cgh.2019.07.018
Durability of Spontaneous and Treatment-Related Loss of Hepatitis B s Antigen
Abstract
Background & aims: Clearance of hepatitis B surface antigen (HBsAg) from serum is the most desirable end point and a proposed definition of functional cure for hepatitis B virus (HBV) infection. However, little is known about the long-term durability of HBsAg loss, and there is controversy over whether the development of antibodies against HBsAg (anti-HBs) is required for maintenance. We aimed to assess the durability of spontaneous or treatment-related (interferon or nucleos(t)ide analogue [NA]) loss of HBsAg.
Methods: We performed a retrospective study of patients with chronic HBV infection followed up at the National Institutes of Health from February 1980 through November 2017. We identified those with HBsAg loss, confirmed on 2 visits at least 24 weeks apart. Patients with hepatitis C virus, hepatitis D virus, human immunodeficiency virus, or human T lymphocyte virus co-infection or HBsAg loss after liver transplantation were excluded. Patients were assigned to the following groups: spontaneous clearance (cleared HBsAg without ever receiving treatment or those who received treatment with a NA or interferon and discontinued therapy >5 years before HBsAg loss), interferon-treated (cleared HBsAg either during treatment or ≤5 years after stopping interferon), and NA-treated (cleared HBsAg either during treatment or ≤5 years after stopping NA).
Results: Among the 787 HBsAg-positive patients, 89 achieved HBsAg loss; 65 of 89 had confirmed HBsAg loss, which was spontaneous in 19 of the patients (29%), after interferon in 22 (34%), and after NA in 24 (37%). Of the 65 patients with confirmed loss of HBsAg, 62 patients (95%) remained HBsAg negative after a mean time of 9.6 years from the first negative HBsAg test result. HBsAg seroreversion occurred in 3 of the 46 treated patients (7%) (1 interferon and 2 NA), 1 of whom was positive for anti-HBs. At the time of HBsAg loss, 33 of 65 (51%) were anti-HBs positive. At the last follow-up evaluation, anti-HBs was detectable in 50 of the 62 patients (81%) assessed. The rate of development of anti-HBs was proportionally higher among interferon-treated patients (19 of 21; 90%) than NA-treated patients (17 of 22; 77%) or patients with spontaneous loss of HBsAg (14 of 19; 74%).
Conclusions: In a retrospective study of 787 HBsAg-positive patients, loss of HBsAg (either spontaneous or after treatment) was confirmed in 8% and was durable. Seroconversion to anti-HBs increased over time and appeared to be more frequent after interferon treatment. HBsAg loss is therefore a robust end point for functional cure.
Keywords: CHB; Outcome; Seroconversion; Vaccination.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures






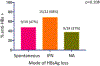

Comment in
-
How Do We Determine Whether a Functional Cure for HBV Infection Has Been Achieved?Clin Gastroenterol Hepatol. 2020 Mar;18(3):548-550. doi: 10.1016/j.cgh.2019.08.033. Epub 2019 Aug 22. Clin Gastroenterol Hepatol. 2020. PMID: 31446185 No abstract available.
References
-
- McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009;49:S45–55. - PubMed
-
- Yuen MF, Wong DK, Fung J, et al. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology 2008;135:1192–9. - PubMed
-
- Liu J, Yang HI, Lee MH, et al. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut 2014;63:1648–57. - PubMed
-
- Yip TC, Wong GL, Chan HL, et al. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J Hepatol 2019;70:361–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

