Suppression of Brown Adipocyte Autophagy Improves Energy Metabolism by Regulating Mitochondrial Turnover
- PMID: 31323770
- PMCID: PMC6678363
- DOI: 10.3390/ijms20143520
Suppression of Brown Adipocyte Autophagy Improves Energy Metabolism by Regulating Mitochondrial Turnover
Abstract
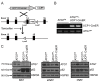
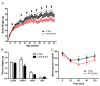
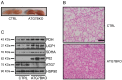
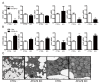
The high abundance of mitochondria and the expression of mitochondrial uncoupling protein 1 (UCP1) confer upon brown adipose tissue (BAT) the unique capacity to convert chemical energy into heat at the expense of ATP synthesis. It was long believed that BAT is present only in infants, and so, it was not considered as a potential therapeutic target for metabolic syndrome; however, the discovery of metabolically active BAT in adult humans has re-stimulated interest in the contributions of BAT metabolic regulation and dysfunction to health and disease. Here we demonstrate that brown adipocyte autophagy plays a critical role in the regulation BAT activity and systemic energy metabolism. Mice deficient in brown adipocyte autophagy due to BAT-specific deletion of Atg7-a gene essential for autophagosome generation-maintained higher mitochondrial content due to suppression of mitochondrial clearance and exhibited improved insulin sensitivity and energy metabolism. Autophagy was upregulated in BAT of older mice compared to younger mice, suggesting its involvement in the age-dependent decline of BAT activity and metabolic rate. These findings suggest that brown adipocyte autophagy plays a crucial role in metabolism and that targeting this pathway may be a potential therapeutic strategy for metabolic syndrome.
Keywords: aging; autophagy; brown adipose tissues; energy homeostasis; mitophagy.
Conflict of interest statement
The authors declare no conflict of interest
Figures


References
-
- Aquila H., Link T.A., Klingenberg M. The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J. 1985;4:2369–2376. doi: 10.1002/j.1460-2075.1985.tb03941.x. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

