Comparative Radioimmunotherapy of Experimental Melanoma with Novel Humanized Antibody to Melanin Labeled with 213Bismuth and 177Lutetium
- PMID: 31323785
- PMCID: PMC6680821
- DOI: 10.3390/pharmaceutics11070348
Comparative Radioimmunotherapy of Experimental Melanoma with Novel Humanized Antibody to Melanin Labeled with 213Bismuth and 177Lutetium
Abstract
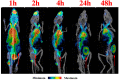
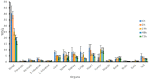
Melanoma is a cancer with increasing incidence and there is a need for alternatives to immunotherapy within effective approaches to treatment of metastatic melanoma. We performed comparative radioimmunotherapy (RIT) of experimental B16-F10 melanoma with novel humanized IgG to melanin h8C3 labeled with a beta emitter, 177Lu, and an alpha-emitter, 213Bi, as well as biodistribution, microSPECT/CT imaging, and mouse and human dosimetry calculations. microSPECT/CT imaging showed that a humanized antibody that targets "free" melanin in the tumor microenvironment had high tumor uptake in B16F10 murine melanoma in C57Bl/6 mice, with little to no uptake in naturally melanized tissues. Extrapolation of the mouse dosimetry data to an adult human demonstrated that doses delivered to major organs and the whole body by 177Lu-h8C3 would be approximately two times higher than those delivered by 213Bi-h8C3, while the doses to the tumor would be almost similar. RIT results indicated that 213Bi-h8C3 was more effective in slowing down the tumor growth than 177Lu-h8C3, while both radiolabeled antibodies did not produce significant hematologic or systemic side effects. We concluded that h8C3 antibody labeled with 213Bi is a promising reagent for translation into a clinical trial in patients with metastatic melanoma.
Keywords: 177Lutetium; 213Bismuth; B16-F10 melanoma; humanized antibody; melanin; radioimmunotherapy.
Conflict of interest statement
E.D. received the research funding from Radimmune Therapeutics. E.D. and D.R. are co-inventors on U.S. Provisional Patent Application No. 62/558,230 MELANIN ANTIBODIES AND USES THEREOF. The RadImmune Therapeutics funder contributed to the design of the study. All other authors declare no conflict of interest.
Figures

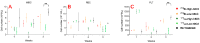
Similar articles
-
A Theranostic Approach to Imaging and Treating Melanoma with 203Pb/212Pb-Labeled Antibody Targeting Melanin.Cancers (Basel). 2023 Jul 29;15(15):3856. doi: 10.3390/cancers15153856. Cancers (Basel). 2023. PMID: 37568672 Free PMC article.
-
Evaluating the Combination of Radioimmunotherapy and Immunotherapy in a Melanoma Mouse Model.Int J Mol Sci. 2020 Jan 24;21(3):773. doi: 10.3390/ijms21030773. Int J Mol Sci. 2020. PMID: 31991626 Free PMC article.
-
Mechanistic Insights into Synergy between Melanin-Targeting Radioimmunotherapy and Immunotherapy in Experimental Melanoma.Int J Mol Sci. 2020 Nov 18;21(22):8721. doi: 10.3390/ijms21228721. Int J Mol Sci. 2020. PMID: 33218169 Free PMC article.
-
Targeting Melanin in Melanoma with Radionuclide Therapy.Int J Mol Sci. 2022 Aug 23;23(17):9520. doi: 10.3390/ijms23179520. Int J Mol Sci. 2022. PMID: 36076924 Free PMC article. Review.
-
Targeted Radionuclide Therapy of Melanoma.Semin Nucl Med. 2016 May;46(3):250-9. doi: 10.1053/j.semnuclmed.2015.12.005. Semin Nucl Med. 2016. PMID: 27067506 Review.
Cited by
-
A Theranostic Approach to Imaging and Treating Melanoma with 203Pb/212Pb-Labeled Antibody Targeting Melanin.Cancers (Basel). 2023 Jul 29;15(15):3856. doi: 10.3390/cancers15153856. Cancers (Basel). 2023. PMID: 37568672 Free PMC article.
-
Radioimmunotherapy as a pathogen-agnostic treatment method for opportunistic mucormycosis infections.Access Microbiol. 2023 Dec 6;5(12):000671.v4. doi: 10.1099/acmi.0.000671.v4. eCollection 2023. Access Microbiol. 2023. PMID: 38188245 Free PMC article.
-
Current landscape and future directions of targeted-alpha-therapy for glioblastoma treatment.Theranostics. 2025 Mar 31;15(11):4861-4889. doi: 10.7150/thno.106081. eCollection 2025. Theranostics. 2025. PMID: 40303349 Free PMC article. Review.
-
Anti-tumor efficacy of a combination therapy with PD-L1 targeted alpha therapy and adoptive cell transfer of PD-1 deficient melanoma-specific human T-lymphocytes.Oncoimmunology. 2021 Jun 27;10(1):1940676. doi: 10.1080/2162402X.2021.1940676. eCollection 2021. Oncoimmunology. 2021. PMID: 34239774 Free PMC article.
-
Radiolabelling small and biomolecules for tracking and monitoring.RSC Adv. 2022 Nov 11;12(50):32383-32400. doi: 10.1039/d2ra06236d. eCollection 2022 Nov 9. RSC Adv. 2022. PMID: 36425706 Free PMC article. Review.
References
-
- American Cancer Society Skin Cancer—Melanoma. [(accessed on 22 June 2019)]; Available online: http://www.cancer.org/cancer/skincancer-melanoma/detailedguide/melanoma-....
-
- Schadendorf D., Hodi F.S., Robert C., Weber J.S., Margolin K., Hamid O., Patt D., Chen T.T., Berman D.M., Wolchok J.D. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

