Differential Roles of Dendritic Cells in Expanding CD4 T Cells in Sepsis
- PMID: 31323786
- PMCID: PMC6783955
- DOI: 10.3390/biomedicines7030052
Differential Roles of Dendritic Cells in Expanding CD4 T Cells in Sepsis
Abstract
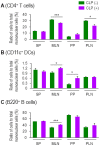
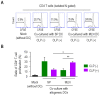
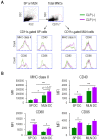
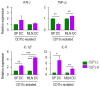
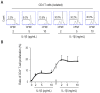
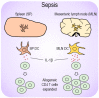
Sepsis is a systemically dysregulated inflammatory syndrome, in which dendritic cells (DCs) play a critical role in coordinating aberrant immunity. The aim of this study is to shed light on the differential roles played by systemic versus mucosal DCs in regulating immune responses in sepsis. We identified a differential impact of the systemic and mucosal DCs on proliferating allogenic CD4 T cells in a mouse model of sepsis. Despite the fact that the frequency of CD4 T cells was reduced in septic mice, septic mesenteric lymph node (MLN) DCs proved superior to septic spleen (SP) DCs in expanding allogeneic CD4 T cells. Moreover, septic MLN DCs markedly augmented the surface expression of MHC class II and CD40, as well as the messaging of interleukin-1β (IL-1β). Interestingly, IL-1β-treated CD4 T cells expanded in a dose-dependent manner, suggesting that this cytokine acts as a key mediator of MLN DCs in promoting septic inflammation. Thus, mucosal and systemic DCs were found to be functionally different in the way CD4 T cells respond during sepsis. Our study provides a molecular basis for DC activity, which can be differential in nature depending on location, whereby it induces septic inflammation or immune-paralysis.
Keywords: CD4 T cells; IL-1β; dendritic cells; mesenteric lymph nodes; sepsis; spleen.
Conflict of interest statement
The authors declare that they have no conflicts of interest with respect to the contents of this article. The funding sponsors had no role in the design of the study, collection, analyses, interpretation of data, writing of the manuscript, or the decision to publish the data.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

