Short Tandem Repeat Expansions and RNA-Mediated Pathogenesis in Myotonic Dystrophy
- PMID: 31323950
- PMCID: PMC6651174
- DOI: 10.3390/ijms20133365
Short Tandem Repeat Expansions and RNA-Mediated Pathogenesis in Myotonic Dystrophy
Abstract
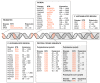
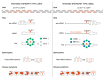
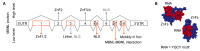
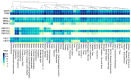
Short tandem repeat (STR) or microsatellite, expansions underlie more than 50 hereditary neurological, neuromuscular and other diseases, including myotonic dystrophy types 1 (DM1) and 2 (DM2). Current disease models for DM1 and DM2 propose a common pathomechanism, whereby the transcription of mutant DMPK (DM1) and CNBP (DM2) genes results in the synthesis of CUG and CCUG repeat expansion (CUGexp, CCUGexp) RNAs, respectively. These CUGexp and CCUGexp RNAs are toxic since they promote the assembly of ribonucleoprotein (RNP) complexes or RNA foci, leading to sequestration of Muscleblind-like (MBNL) proteins in the nucleus and global dysregulation of the processing, localization and stability of MBNL target RNAs. STR expansion RNAs also form phase-separated gel-like droplets both in vitro and in transiently transfected cells, implicating RNA-RNA multivalent interactions as drivers of RNA foci formation. Importantly, the nucleation and growth of these nuclear foci and transcript misprocessing are reversible processes and thus amenable to therapeutic intervention. In this review, we provide an overview of potential DM1 and DM2 pathomechanisms, followed by a discussion of MBNL functions in RNA processing and how multivalent interactions between expanded STR RNAs and RNA-binding proteins (RBPs) promote RNA foci assembly.
Keywords: ALS/FTD; CELF; MBNL; RBFOX; STR; alternative splicing; foci; microsatellite expansion; myotonic dystrophy; phase separation.
Conflict of interest statement
M.S.S. is a member of the scientific advisory board of Locana Bio, Inc. Ł.J.S. declares no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

