Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments
- PMID: 31324218
- PMCID: PMC6642605
- DOI: 10.1186/s40425-019-0666-1
Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments
Abstract
Background: Innate immune cells such as macrophages are abundantly present within malignant ascites, where they share the microenvironment with ovarian cancer stem cells (CSC).
Methods: To mimic this malignant ascites microenvironment, we created a hanging-drop hetero-spheroid model to bring CSCs and macrophages in close association. Within these hetero-spheroids, CD68+ macrophages (derived from U937 or peripheral blood monocytes) make up ~ 20% of the population, while the rest are ovarian cancer cells and ovarian cancer stem cells (derived from the high grade serous ovarian cancer cell line, OVCAR3).
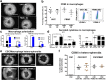
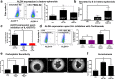
Results: Our results indicate that CSCs drive the upregulation of M2 macrophage marker CD206 within hetero-spheroids, compared to bulk ovarian cancer cells, implying an inherently more immuno-suppressive program. Moreover, an increased maintenance of elevated aldehyde dehydrogenase (ALDH) activity is noted within hetero-spheroids that include pre-polarized CD206+ M2 macrophages, implying a reciprocal interaction that drives pro-tumoral activation as well as CSC self-renewal. Consistent with enriched CSCs, we also observe increased levels of pro-tumoral IL-10 and IL-6 cytokines in the CSC/M2-macrophage hetero-spheroids. CSC/M2-macrophage hetero-spheroids are also less sensitive to the chemotherapeutic agent carboplatin and are subsequently more invasive in transwell assays. Using inhibitors of WNT secretion in both CSCs and macrophages, we found that CSC-derived WNT ligands drove CD206+ M2 macrophage activation, and that, conversely, macrophage-derived WNT ligands enriched ALDH+ cells within the CSC compartment of hetero-spheroids. Upon examination of specific WNT ligand expression within the monocyte-derived macrophage system, we observed a significant elevation in gene expression for WNT5B. In CSCs co-cultured with macrophages within hetero-spheroids, increases in several WNT ligands were observed, and this increase was significantly inhibited when WNT5B was knocked down in macrophages.
Conclusions: Our data implies that macrophage- initiated WNT signaling could play a significant role in the maintenance of stemness, and the resulting phenotypes of chemoresistance and invasiveness. Our results indicate paracrine WNT activation during CSC/M2 macrophages interaction constitutes a positive feedback loop that likely contributes to the more aggressive phenotype, which makes the WNT pathway a potential target to reduce the CSC and M2 macrophage compartments in the tumor microenvironment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Allen HJ, Porter C, Gamarra M, Piver MS, Johnson EA. Isolation and morphologic characterization of human ovarian carcinoma cell clusters present in effusions. Exp Cell Biol. 1987;55:194–208. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
