Dendritic spine geometry and spine apparatus organization govern the spatiotemporal dynamics of calcium
- PMID: 31324651
- PMCID: PMC6683673
- DOI: 10.1085/jgp.201812261
Dendritic spine geometry and spine apparatus organization govern the spatiotemporal dynamics of calcium
Erratum in
-
Addendum: Dendritic spine geometry and spine apparatus organization govern the spatiotemporal dynamics of calcium.J Gen Physiol. 2019 Sep 2;151(9):2221-2222. doi: 10.1085/jgp.20181226107312019a. Epub 2019 Aug 2. J Gen Physiol. 2019. PMID: 31375548 Free PMC article. No abstract available.
Abstract
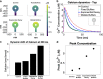
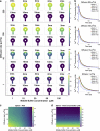
Dendritic spines are small subcompartments that protrude from the dendrites of neurons and are important for signaling activity and synaptic communication. These subcompartments have been characterized to have different shapes. While it is known that these shapes are associated with spine function, the specific nature of these shape-function relationships is not well understood. In this work, we systematically investigated the relationship between the shape and size of both the spine head and spine apparatus, a specialized endoplasmic reticulum compartment within the spine head, in modulating rapid calcium dynamics using mathematical modeling. We developed a spatial multicompartment reaction-diffusion model of calcium dynamics in three dimensions with various flux sources, including N-methyl-D-aspartate receptors (NMDARs), voltage-sensitive calcium channels (VSCCs), and different ion pumps on the plasma membrane. Using this model, we make several important predictions. First, the volume to surface area ratio of the spine regulates calcium dynamics. Second, membrane fluxes impact calcium dynamics temporally and spatially in a nonlinear fashion. Finally, the spine apparatus can act as a physical buffer for calcium by acting as a sink and rescaling the calcium concentration. These predictions set the stage for future experimental investigations of calcium dynamics in dendritic spines.
© 2019 Bell et al.
Figures







Comment in
-
How dendritic spines shape calcium dynamics.J Gen Physiol. 2019 Aug 5;151(8):970. doi: 10.1085/jgp.201912432. Epub 2019 Jul 19. J Gen Physiol. 2019. PMID: 31324650 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

