Inclusion of Older Patients with Cancer in Clinical Trials: The SAGE Prospective Multicenter Cohort Survey
- PMID: 31324663
- PMCID: PMC6975930
- DOI: 10.1634/theoncologist.2019-0166
Inclusion of Older Patients with Cancer in Clinical Trials: The SAGE Prospective Multicenter Cohort Survey
Abstract
Background: The primary objective was to evaluate the rates of older patients with colorectal cancer (CRC) who were eligible for a clinical trial, invited to participate, and, ultimately, included. The secondary objective was to assess the reasons for ineligibility, noninvitation, and noninclusion and factors associated.
Materials and methods: The Sujets AGés dans les Essais Cliniques (SAGE; Older Subjects in Clinical Trials) multicenter prospective cohort was established in seven centers (10 departments of medical oncology, digestive oncology, and digestive surgery) between 2012 and 2016. All patients with CRC aged 65 or older were studied. The endpoints were clinical trial availability, patient's eligibility, invitation, and enrollment in a trial.
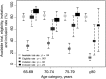
Results: We included 577 older patients (mean age ± SD: 75.6 ± 7 years; males: 56%; metastasis: 41%). Thirty-seven trials were ongoing (one trial for older patients). Of the 474 patients with at least one available trial for their cancer stage and site, 127 (27%) were eligible; 84 of these 127 (66%) were invited to participate, and 70 of these 84 (83%) were included. In a multivariate analysis, noninvitation was found to be associated with older age (p = .016): adjusted relative risk (95% confidence interval), 0.14 (0.02-0.60) for ≥80 vs. 65-69; 0.54 (0.18-1.04) for 75-79 vs. 65-69; 0.47 (0.17-0.93) for 70-74 vs. 65-69.
Conclusion: Three-quarters of older patients with CRC were ineligible for a clinical trial. One-third of the eligible patients were not invited to participate in a trial, and 17% of invited patients were not included. Few trials are reserved for older patients. Patients aged 80 or older were significantly less likely to be eligible for a trial and invited to participate. Clinical trial identification number: NCT01754636.
Implications for practice: The results of this study suggest that barriers to participation of older patients in clinical trials are particularly marked at age 80 years or older. Secondly, the results emphasize the need for trials for older patients. Thirdly, there is also a need for more pragmatic "real-world" trials, rather than solely randomized trials performed in idealized settings with strictly selected patients. Large prospective observational cohorts with a precise follow-up of toxicity, functional decline, and quality of life may constitute one way of generating more data on the risk-benefit ratio for cancer treatments in older patients.
Keywords: Clinical trial; Colorectal cancer; Eligibility; Inclusion; Older patient; Over‐80.
© AlphaMed Press 2019.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Dechartres A, Chevret S, Lambert J et al. Inclusion of patients with acute leukemia in clinical trials: A prospective multicenter survey of 1066 cases. Ann Oncol 2011;22:224–233. - PubMed
-
- Gouverneur A, Salvo F, Berdaï D et al. Inclusion of elderly or frail patients in randomized controlled trials of targeted therapies for the treatment of metastatic colorectal cancer: A systematic review. J Geriatr Oncol 2018;9:15–23. - PubMed
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race‐, sex‐, and age‐based disparities. JAMA 2004;291:2720–2726. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical