Embryonic FAP+ lymphoid tissue organizer cells generate the reticular network of adult lymph nodes
- PMID: 31324739
- PMCID: PMC6780995
- DOI: 10.1084/jem.20181705
Embryonic FAP+ lymphoid tissue organizer cells generate the reticular network of adult lymph nodes
Abstract
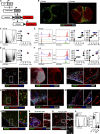
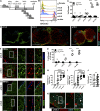
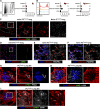
The induction of adaptive immunity is dependent on the structural organization of LNs, which is in turn governed by the stromal cells that underpin LN architecture. Using a novel fate-mapping mouse model, we trace the developmental origin of mesenchymal LN stromal cells (mLNSCs) to a previously undescribed embryonic fibroblast activation protein-α (FAP)+ progenitor. FAP+ cells of the LN anlagen express lymphotoxin β receptor (LTβR) and vascular cell adhesion molecule (VCAM), but not intercellular adhesion molecule (ICAM), suggesting they are early mesenchymal lymphoid tissue organizer (mLTo) cells. Clonal labeling shows that FAP+ progenitors locally differentiate into mLNSCs. This process is also coopted in nonlymphoid tissues in response to infection to facilitate the development of tertiary lymphoid structures, thereby mimicking the process of LN ontogeny in response to infection.
© 2019 Denton et al.
Figures



References
-
- Baddeley A., and Turner R.. 2005. spatstat: An R package for analyzing spatial point patterns. J. Stat. Softw. 12:1–42. 10.18637/jss.v012.i06 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

