Human immune globulin 10% with recombinant human hyaluronidase in multifocal motor neuropathy
- PMID: 31325017
- PMCID: PMC6803588
- DOI: 10.1007/s00415-019-09475-x
Human immune globulin 10% with recombinant human hyaluronidase in multifocal motor neuropathy
Abstract
Objective: The primary aim was to determine the safety of treatment with human immune globulin 10% with recombinant human hyaluronidase (fSCIg) compared to intravenous immunoglobulin (IVIg) in a prospective open-label study in patients with multifocal motor neuropathy (MMN).
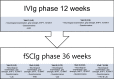
Methods: Our study consisted of two phases: the IVIg phase (visits 1-3; 12 weeks), in which patients remained on IVIg treatment, and the fSCIg phase (visits 4-7; 36 weeks), in which patients received fSCIg treatment. After visit 3, IVIg was switched to an equivalent dose and frequency of fSCIg. Outcome measures were safety, muscle strength, disability and treatment satisfaction.
Results: Eighteen patients were enrolled in this study. Switching to fSCIg reduced the number of systemic adverse events (IVIg 11.6 vs. fSCIg 5.0 adverse events/per person-year, p < 0.02), and increased the number of local reactions at the injection site (IVIg 0 vs. fSCIg 3.3 local reactions/per person-year, p < 0.01). Overall, no significant differences in muscle strength and disability between fSCIg and IVIg were found. Treatment with fSCIg was perceived as optimal treatment option by 8 of the 17 patients (47.1%) and they continued with fSCIg after study closure because of improved independence and flexibility to administer treatment.
Conclusion: Treatment with fSCIg can be considered a safe alternative for patients with MMN on IVIg treatment. fSCIg could be a favorable option in patients who prefer self-treatment and more independency, and in patients who experience systemic adverse events on IVIg or have difficult intravenous access.
Keywords: Immunoglobulin; Multifocal motor neuropathy; Peripheral neuropathy; Safety; Treatment effect.
Conflict of interest statement
IH, JB, RE—these authors declare that they have no conflict of interest. SG—receives research support from the Prinses Beatrix Spierfonds, and received a travel grant and speaker fee from Shire/Takeda. LP—receives research support from the Prinses Beatrix Spierfonds, and Stichting Spieren voor Spieren. LB—serves on scientific advisory boards for Orion, Biogen, and Cytokinetics; received an educational grant from Baxalta/Takeda; serves on the editorial boards of Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration and the Journal of Neurology, Neurosurgery, and Psychiatry; and receives research support from the Prinses Beatrix Spierfonds, Netherlands ALS Foundation, The European Community’s Health Seventh Framework Programme (Grant agreement no. 259867), and The Netherlands Organization for Health Research and Development (Vici Scheme, JPND [SOPHIA, STRENGTH, ALSCare]).
Figures
References
-
- Joint Task Force of the E, the PNS (2010) European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society--first revision. J Peripher Nerv Syst 15 (4):295–301. doi: 10.1111/j.1529–8027.2010.00290. - PubMed
-
- van Schaik IN, van den Berg LH, de Haan R, Vermeulen M (2005) Intravenous immunoglobulin for multifocal motor neuropathy. Cochrane Database Syst Rev 2:CD004429. doi: 10.1002/14651858.CD004429.pub2 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

