Heme biosynthesis and the porphyrias
- PMID: 31326287
- PMCID: PMC7252266
- DOI: 10.1016/j.ymgme.2019.04.008
Heme biosynthesis and the porphyrias
Abstract
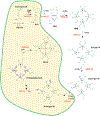
Porphyrias, is a general term for a group of metabolic diseases that are genetic in nature. In each specific porphyria the activity of specific enzymes in the heme biosynthetic pathway is defective and leads to accumulation of pathway intermediates. Phenotypically, each disease leads to either neurologic and/or photocutaneous symptoms based on the metabolic intermediate that accumulates. In each porphyria the distinct patterns of these substances in plasma, erythrocytes, urine and feces are the basis for diagnostically defining the metabolic defect underlying the clinical observations. Porphyrias may also be classified as either erythropoietic or hepatic, depending on the principal site of accumulation of pathway intermediates. The erythropoietic porphyrias are congenital erythropoietic porphyria (CEP), and erythropoietic protoporphyria (EPP). The acute hepatic porphyrias include ALA dehydratase deficiency porphyria, acute intermittent porphyria (AIP), hereditary coproporphyria (HCP) and variegate porphyria (VP). Porphyria cutanea tarda (PCT) is the only porphyria that has both genetic and/or environmental factors that lead to reduced activity of uroporphyrinogen decarboxylase in the liver. Each of the 8 enzymes in the heme biosynthetic pathway have been associated with a specific porphyria (Table 1). Mutations affecting the erythroid form of ALA synthase (ALAS2) are most commonly associated with X-linked sideroblastic anemia, however, gain-of-function mutations of ALAS2 have also been associated with a variant form of EPP. This overview does not describe the full clinical spectrum of the porphyrias, but is meant to be an overview of the biochemical steps that are required to make heme in both erythroid and non-erythroid cells.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
References
-
- Granick S, Sassa S, δ-aminolevulinic acid synthase and the control of heme and chlorophyll synthesis, in: Vogel HJ (Ed.), Metabolic Regulation, Academic Press, New York, 1971, p. 77.
-
- Sassa S, Kappas A, Genetic, metabolic and biochemical aspects of the porphyrias, in: Harris H, Hirschhorn K (Eds.), AdHum Genet, Plenum Publications, New York, 1981, p. 121. - PubMed
-
- Corcos L, Phenobarbital and dexamethasone induce expression of cytochrome P450 genes from subfamilies IIB, IIC, and IIIA in mouse liver, Drug Metab. Dispos 20 (6) (1992) 797–801. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

