Notch signaling in breast cancer: From pathway analysis to therapy
- PMID: 31326555
- PMCID: PMC9003668
- DOI: 10.1016/j.canlet.2019.07.012
Notch signaling in breast cancer: From pathway analysis to therapy
Abstract
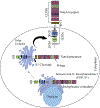
The Notch signaling pathway, which is highly conserved from sea urchins to humans, plays an important role in cell-differentiation, survival, proliferation, stem-cell renewal, and determining cell fate during development and morphogenesis. It is well established that signaling pathways are dysregulated in a wide-range of diseases, including human malignancies. Studies suggest that the dysregulation of the Notch pathway contributes to carcinogenesis, cancer stem cell renewal, angiogenesis, and chemo-resistance. Elevated levels of Notch receptors and ligands have been associated with cancer-progression and poor survival. Furthermore, the Notch signaling pathway regulates the transcriptional activity of key target genes through crosstalk with several other signaling pathways. Indeed, increasing evidence suggests that the Notch signaling pathway may serve as a therapeutic target for the treatment of several cancers, including breast cancer. Researchers have demonstrated the anti-tumor properties of Notch inhibitors in various cancer types. Currently, Notch inhibitors are being evaluated for anticancer efficacy in a number of clinical-trials. However, because there are multiple Notch receptors that can exhibit either oncogenic or tumor-suppressing roles in various cells, it is important that the Notch inhibitors are specific to particular receptors that are tumorigenic in nature. This review critically evaluates existing Notch inhibitory drugs and strategies and summarizes the previous discoveries, current understandings, and recent developments in support of Notch receptors as therapeutic targets in breast cancer.
Keywords: Breast cancer; Chemo-resistance; Monoclonal antibodies; Notch receptor; γ-secretase inhibitors.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

References
-
- Theodosiou A, Arhondakis S, Baumann M, Kossida S. Evolutionary scenarios of Notch proteins. Mol Biol Evol 2009; 26: 1631–40. - PubMed
-
- Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis-Tsakonas S. Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell 1991; 67: 687–99. - PubMed
-
- Weinmaster G, Roberts VJ, Lemke G. Notch2: a second mammalian Notch gene. Development 1992; 116: 931–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

