Interactions between the complement and endothelin systems in normal pregnancy and following placental ischemia
- PMID: 31326653
- PMCID: PMC6774867
- DOI: 10.1016/j.molimm.2019.06.015
Interactions between the complement and endothelin systems in normal pregnancy and following placental ischemia
Abstract
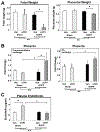
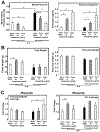
Preeclampsia is characterized by new onset hypertension and fetal growth restriction and is associated with aberrant activation of the innate immune complement system and stressed or ischemic placenta. Previous studies have suggested a role for both endothelin and complement system activation products in new onset hypertension in pregnancy, but inter-relationships of the pathways are unclear. We hypothesized that complement activation following placental ischemia stimulates the endothelin pathway to cause hypertension and impair fetal growth. The Reduced Uterine Perfusion Pressure (RUPP) model results in hypertension and fetal growth restriction in a pregnant rat due to placental ischemia caused by mechanical obstruction of blood flow to uterus and placenta. The effect of inhibitor of complement activation soluble Complement Receptor 1 (sCR1) and endothelin A receptor (ETA) antagonist atrasentan on hypertension, fetal weight, complement activation (systemic circulating C3a and local C3 placental deposition) and endothelin [circulating endothelin and message for preproendothelin (PPE), ETA and endothelin B receptor (ETB) in placenta] in the RUPP rat model were determined. Following placental ischemia, sCR1 attenuated hypertension but increased message for PPE and ETA in placenta, suggesting complement activation causes hypertension via an endothelin independent pathway. With ETA antagonism the placental ischemia-induced increase in circulating C3a was unaffected despite inhibition of hypertension, indicating systemic C3a alone is not sufficient. In normal pregnancy, inhibiting complement activation increased plasma endothelin but not placental PPE message. Atrasentan treatment increased fetal weight, circulating endothelin and placental ETA message, and unexpectedly increased local complement activation in placenta (C3 deposition) but not C3a in circulation, suggesting endothelin controls local placental complement activation in normal pregnancy. Atrasentan also significantly decreased message for endogenous complement regulators Crry and CD55 in placenta and kidney in normal pregnancy. Results of our study indicate that complement/endothelin interactions differ in pregnancies complicated with placental ischemia vs normal pregnancy, as well as locally vs systemically. These data clearly illustrate the complex interplay between complement and endothelin indicating that perturbations of either pathway may affect pregnancy outcomes.
Keywords: Complement; Endothelin; Placental ischemia; Pregnancy.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosures or Conflict of Interest
The authors have no conflicts to report
Figures
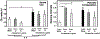

References
-
- Kolev M, Le Friec G, Kemper C, Complement - tapping into new sites and effector systems, Nat Rev Immunol, 14 (2014) 811–820. - PubMed
-
- Derzsy Z, Prohaszka Z, Rigo J Jr., Fust G, Molvarec A, Activation of the complement system in normal pregnancy and preeclampsia, Mol Immunol, 47 (2010) 1500–1506. - PubMed
-
- Burwick RM, Feinberg BB, Eculizumab for the treatment of preeclampsia/HELLP syndrome, Placenta, 34 (2013) 201–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

