Does nociception monitor-guided anesthesia affect opioid consumption? A systematic review of randomized controlled trials
- PMID: 31327102
- PMCID: PMC7367908
- DOI: 10.1007/s10877-019-00362-4
Does nociception monitor-guided anesthesia affect opioid consumption? A systematic review of randomized controlled trials
Abstract
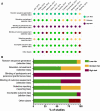
Monitors that estimate nociception during anesthesia may be used to guide opioid and other analgesics administration to optimize anesthesia care and possibly outcome. We reviewed the literature to evaluate current evidence of the effect of nociception-guided management over standard anesthesia practice during surgery. A systematic review of the literature on the effect of nociception monitoring on anesthesia practice was conducted. Reports were eligible if they compared nociception-guided anesthesia to standard practice during surgery. Primary endpoint of this review is intraoperative opioid consumption. Secondary endpoints included hemodynamic control, postoperative pain and pain treatment. We identified 12 randomized controlled trials that compared one of five different nociception monitoring techniques to standard anesthesia care. Most studies were single center studies of small sample size. Six studies reported intraoperative opioid consumption as primary outcome. There was considerable variability with respect to surgical procedure and anesthesia technique. For nociception monitors that were investigated by more than one study, analysis of the pooled data was performed. The surgical plethysmographic index was the only monitor for which an intra operative opioid sparing effect was found. For the other monitors, either no effect was detected, or pooled analysis could not be performed due to paucity of study data. On secondary outcomes, no consistent effect of nociception-guided anesthesia could be established. Although some nociception monitors show promising results, no definitive conclusions regarding the effect of nociception monitoring on intraoperative opioid consumption or other anesthesia related outcome can be drawn.Clinical trial number PROSPERO ID 102913.
Keywords: Nociception monitoring; Opioid consumption; Systematic review.
Conflict of interest statement
AD received Speaker Fee from Medasense Biometrics Ltd. (Israel); RE and DIS serve on an Advisory Board for Medasense Biometrics Ltd. and have an Equity Interest in the company. Medasense is the Developer of the nociception level index. The other authors declare no competing interests.
Figures

References
-
- Funcke S, Sauerlaender S, Pinnschmidt HO, Saugel B, Bremer K, Reuter DA, Nitzschke R. Validation of innovative techniques for monitoring nociception during general anesthesia: a clinical study using tetanic and intracutaneous electrical stimulation. Anesthesiology. 2017;127(2):272–283. doi: 10.1097/aln.0000000000001670. - DOI - PubMed
-
- Stockle PA, Julien M, Issa R, Decary E, Brulotte V, Drolet P, Henri M, Poirier M, Latulippe JF, Dorais M, Verdonck O, Fortier LP, Richebe P. Validation of the PMD100 and its NOL Index to detect nociception at different infusion regimen of remifentanil in patients under general anesthesia. Minerva Anestesiol. 2018;84(10):1160–1168. doi: 10.23736/s0375-9393.18.12720-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

