A Single MicroRNA-Hox Gene Module Controls Equivalent Movements in Biomechanically Distinct Forms of Drosophila
- PMID: 31327720
- PMCID: PMC6710004
- DOI: 10.1016/j.cub.2019.06.082
A Single MicroRNA-Hox Gene Module Controls Equivalent Movements in Biomechanically Distinct Forms of Drosophila
Abstract
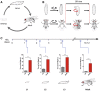
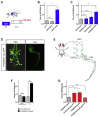
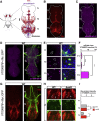
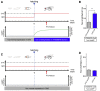
Movement is the main output of the nervous system. It emerges during development to become a highly coordinated physiological process essential to survival and adaptation of the organism to the environment. Similar movements can be observed in morphologically distinct developmental stages of an organism, but it is currently unclear whether or not these movements have a common molecular cellular basis. Here we explore this problem in Drosophila, focusing on the roles played by the microRNA (miRNA) locus miR-iab4/8, which we previously showed to be essential for the normal corrective response displayed by the fruit fly larva when turned upside down (self-righting). Our study shows that miR-iab4 is required for normal self-righting across all three Drosophila larval stages. Unexpectedly, we also discover that this miRNA is essential for normal self-righting behavior in the adult fly, an organism with different morphology, neural constitution, and biomechanics. Through the combination of gene expression, optical imaging, and quantitative behavioral approaches, we provide evidence that miR-iab4 exerts its effects on adult self-righting behavior in part through repression of the Hox gene Ultrabithorax (Ubx) in a specific set of adult motor neurons, the NB2-3/lin15 neurons. Our results show that miRNA controls the function, rather than the morphology, of these neurons and demonstrate that post-developmental changes in Hox gene expression can modulate behavior in the adult. Our work reveals that a common miRNA-Hox genetic module can be re-deployed in different neurons to control functionally equivalent movements in biomechanically distinct organisms and describes a novel post-developmental role of the Hox genes in adult neural function.
Keywords: Drosophila; Hox genes; Ubx; Ultrabithorax; adult/larva; behavior; microRNA; motor neuron; movement; self-righting.
Copyright © 2019 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Sherrington C.S. Yale University Press; 1906. The Integrative Action of the Nervous System, Edition.
-
- Sherrington C.S. Some aspects of animal mechanism. Science. 1922;56:345–355. - PubMed
-
- Saint-Amant L., Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998;37:622–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

