Quality of Life Following Prestige LP Cervical Disc Arthroplasty in a Prospective Multicountry Study
- PMID: 31328085
- PMCID: PMC6625717
- DOI: 10.14444/6030
Quality of Life Following Prestige LP Cervical Disc Arthroplasty in a Prospective Multicountry Study
Abstract
Background: To describe routine surgical practice using Prestige LP Cervical Disc (Prestige disc) and patient outcomes for degenerative cervical disc disease in a multicenter 2-year prospective, observational study.
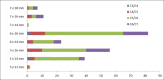
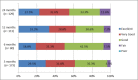
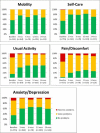
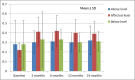
Methods: Patient demographics and intraoperative data were collected; quality of life (QoL) (EQ-5D, EQ-VAS, and neck disability index), average disc height, and adverse events were assessed pre- and postoperatively at 3, 6, 12, and 24 months.
Results: One hundred and ninety-four patients were enrolled (190 patients implanted; female: 67%; mean age: 44.0 years; mean body mass index: 25.6). Disc herniation was the most frequent indication for cervical arthroplasty (80.5%). Thirty-seven percent of patients experienced pain for >1 year prior to baseline assessment. Mean procedure duration was 87.1 minutes, and mean blood loss was 43.8 mL. The majority (71.0%) of Prestige discs were implanted at level C5 to C6, while 16.3% of patients received implants at 2 levels. There was a significant improvement from baseline to 3, 6, 12, and 24 months of follow-up in all QoL assessments. After implantation, the mean disc height at the affected level increased by 0.19 from baseline (0.22) to 3 months (0.41) and remained constant up to 24 months (P < .001). Mean disc height of levels above and below the implant remained comparable at baseline and follow-up. A total of 63 adverse events (44 patients) was recorded, of which 7 (11.1%) were related to the Prestige disc, instrumentation, or procedure; 41 (65.1%) were unrelated; and 15 (23.8%) had an unknown relation.
Conclusions: In line with published findings, our study shows significant improvement in outcomes in the first 3 months after Prestige disc implantation with improvements maintained throughout the study.
Keywords: cervical spine; Prestige LP Cervical Disc; arthroplasty; degenerative disc disease; quality of life.
Conflict of interest statement
Disclosures and COI: Marton Ronai, MD: Dr Rónai reports personal fees from Medtronic Trading NL BV during the conduct of the study; personal fees from Medikomp Kft, outside the submitted work; and travel, hotel, and registration payed by Medtronic for attending Summer University July 1-3, 2015, Vienna, Austria. Dated May 6, 2016. Jiri Matejka, MD: Dr Matejka reports receiving financial support or services from Medtronic during the conduct of the study. Dated May 2, 2016. The study sponsor (Medtronic Clinical Spine, Bakken Research Center BV, The Netherlands) established subcontracts to the participating research sites and/or investigators. Research support for this study was provided by Medtronic Clinical Spine, Bakken Research Center BV, The Netherlands. The study was presented in part at EuroSpine and NASS meetings during 2015. No other conflicts of interest were declared.
Figures
Similar articles
-
Cervical disc arthroplasty with PRESTIGE LP disc versus anterior cervical discectomy and fusion: a prospective, multicenter investigational device exemption study.J Neurosurg Spine. 2015 Nov;23(5):558-573. doi: 10.3171/2015.1.SPINE14589. Epub 2015 Jul 31. J Neurosurg Spine. 2015. PMID: 26230424
-
Long-term clinical and radiographic outcomes of the Prestige LP artificial cervical disc replacement at 2 levels: results from a prospective randomized controlled clinical trial.J Neurosurg Spine. 2017 Jul;27(1):7-19. doi: 10.3171/2016.11.SPINE16746. Epub 2017 Apr 7. J Neurosurg Spine. 2017. PMID: 28387616 Clinical Trial.
-
Clinical and radiographic outcomes of cervical disc arthroplasty with Prestige-LP Disc: a minimum 6-year follow-up study.BMC Musculoskelet Disord. 2018 Aug 7;19(1):285. doi: 10.1186/s12891-018-2201-9. BMC Musculoskelet Disord. 2018. PMID: 30086733 Free PMC article.
-
Cervical disc arthroplasty with Prestige-LP for the treatment of contiguous 2-level cervical degenerative disc disease: 5-year follow-up results.Medicine (Baltimore). 2018 Jan;97(4):e9671. doi: 10.1097/MD.0000000000009671. Medicine (Baltimore). 2018. PMID: 29369186 Free PMC article.
-
Early clinical and biomechanical results following cervical arthroplasty.Neurosurg Focus. 2004 Sep 15;17(3):E9. doi: 10.3171/foc.2004.17.3.9. Neurosurg Focus. 2004. PMID: 15636565 Review.
References
-
- Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2003;28(2):134–139. - PubMed
-
- König SA, Spetzger U. Clinical outcome of anterior cervical discectomy and fusion versus total disc replacement—a meta-analysis of 2532 cases. Neurosurgery. 2016;1(2)
-
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
