Fc receptor-like 1 intrinsically recruits c-Abl to enhance B cell activation and function
- PMID: 31328160
- PMCID: PMC6637015
- DOI: 10.1126/sciadv.aaw0315
Fc receptor-like 1 intrinsically recruits c-Abl to enhance B cell activation and function
Abstract
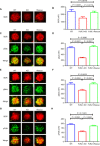
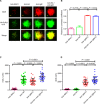
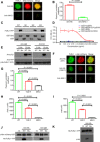
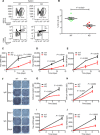
B cell activation is regulated by the stimulatory or inhibitory co-receptors of B cell receptors (BCRs). Here, we investigated the signaling mechanism of Fc receptor-like 1 (FcRL1), a newly identified BCR co-receptor. FcRL1 was passively recruited into B cell immunological synapses upon BCR engagement in the absence of FcRL1 cross-linking, suggesting that FcRL1 may intrinsically regulate B cell activation and function. BCR cross-linking alone led to the phosphorylation of the intracellular Y281ENV motif of FcRL1 to provide a docking site for c-Abl, an SH2 domain-containing kinase. The FcRL1 and c-Abl signaling module, in turn, potently augmented B cell activation and proliferation. FcRL1-deficient mice exhibited markedly impaired formation of extrafollicular plasmablasts and germinal centers, along with decreased antibody production upon antigen stimulation. These findings reveal a critical BCR signal-enhancing function of FcRL1 through its intrinsic recruitment to B cell immunological synapses and subsequent recruitment of c-Abl upon BCR cross-linking.
Figures


References
-
- Miller I., Hatzivassiliou G., Cattoretti G., Mendelsohn C., Dalla-Favera R., IRTAs: A new family of immunoglobulinlike receptors differentially expressed in B cells. Blood 99, 2662–2669 (2002). - PubMed
-
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M., Molecular components of the B-cell antigen receptor complex of the IgM class. Nature 343, 760–762 (1990). - PubMed
-
- Harwood N. E., Batista F. D., Early events in B cell activation. Annu. Rev. Immunol. 28, 185–210 (2010). - PubMed
-
- Reth M., Antigen receptor tail clue. Nature 338, 383–384 (1989). - PubMed
-
- Xu Y., Harder K. W., Huntington N. D., Hibbs M. L., Tarlinton D. M., Lyn tyrosine kinase: Accentuating the positive and the negative. Immunity 22, 9–18 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

