A new case of kleptoplasty in animals: Marine flatworms steal functional plastids from diatoms
- PMID: 31328166
- PMCID: PMC6636991
- DOI: 10.1126/sciadv.aaw4337
A new case of kleptoplasty in animals: Marine flatworms steal functional plastids from diatoms
Abstract
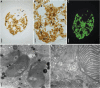
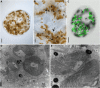
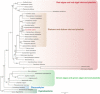
To date, sea slugs have been considered the only animals known to sequester functional algal plastids into their own cells, via a process called "kleptoplasty." We report here, however, that endosymbionts in the marine flatworms Baicalellia solaris and Pogaina paranygulgus are isolated plastids stolen from diatoms. Ultrastructural data show that kleptoplasts are located within flatworm cells, while algal nuclei and other organelles are absent. Transcriptomic analysis and rbcL amplicons confirm the absence of algal nuclear mRNA and reveal that the plastids originate from different species of diatoms. Laboratory experiments demonstrated photosynthetic activity and short-term retention of kleptoplasts in starved worms. This lineage of flatworms represents the first known case of functional kleptoplasty involving diatoms and only the second known case of kleptoplasty across the entire tree of animals.
Figures

References
-
- Venn A. A., Loram J. E., Douglas A. E., Photosynthetic symbioses in animals. J. Exp. Bot. 59, 1069–1080 (2008). - PubMed
-
- Johnson M. D., The acquisition of phototrophy: Adaptive strategies of hosting endosymbionts and organelles. Photosynth. Res. 107, 117–132 (2011). - PubMed
-
- Melo Clavijo J., Donath A., Serôdio J., Christa G., Polymorphic adaptations in metazoans to establish and maintain photosymbioses. Biol. Rev. Camb. Philos. Soc. 93, 2006–2020 (2018). - PubMed
-
- Stoecker D. K., Johnson M. D., de Vargas C., Not F., Acquired phototrophy in aquatic protists. Aquat. Microb. Ecol. 57, 279–310 (2009).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

