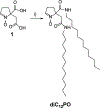
Membrane-specific spin trap, 5-dodecylcarbamoyl-5-N-dodecylacetamide-1-pyroline-N-oxide (diC12PO): theoretical, bioorthogonal fluorescence imaging and EPR studies
- PMID: 31328213
- PMCID: PMC6703941
- DOI: 10.1039/c9ob01334b
Membrane-specific spin trap, 5-dodecylcarbamoyl-5-N-dodecylacetamide-1-pyroline-N-oxide (diC12PO): theoretical, bioorthogonal fluorescence imaging and EPR studies
Abstract
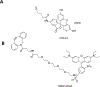
Membranous organelles are major endogenous sources of reactive oxygen and nitrogen species. When present at high levels, these species can cause macromolecular damage and disease. To better detect and scavenge free radical forms of the reactive species at their sources, we investigated whether nitrone spin traps could be selectively targeted to intracellular membranes using a bioorthogonal imaging approach. Electron paramagnetic resonance imaging demonstrated that the novel cyclic nitrone 5-dodecylcarbamoyl-5-N-dodecylacetamide-1-pyroline-N-oxide (diC12PO) could be used to target the nitrone moiety to liposomes composed of phosphatidyl choline. To test localization with authentic membranes in living cells, fluorophores were introduced via strain-promoted alkyne-nitrone cycloaddition (SPANC). Two fluorophore-conjugated alkynes were investigated: hexynamide-fluoresceine (HYA-FL) and dibenzylcyclooctyne-PEG4-5/6-sulforhodamine B (DBCO-Rhod). Computational and mass spectrometry experiments confirmed the cycloadduct formation of DBCO-Rhod (but not HYA-FL) with diC12PO in cell-free solution. Confocal microscopy of bovine aortic endothelial cells treated sequentially with diC12PO and DBCO-Rhod demonstrated clear localization of fluorescence with intracellular membranes. These results indicate that targeting of nitrone spin traps to cellular membranes is feasible, and that a bioorthogonal approach can aid the interrogation of their intracellular compartmentalization properties.
Conflict of interest statement
Conflicts of interest
The authors declare no conflict of interest.
Figures





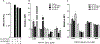




Similar articles
-
Reactive nitrogen species reactivities with nitrones: theoretical and experimental studies.Chem Res Toxicol. 2012 Aug 20;25(8):1581-97. doi: 10.1021/tx200526y. Epub 2012 Jul 31. Chem Res Toxicol. 2012. PMID: 22775566 Free PMC article.
-
Use of spin traps to detect superoxide production in living cells by electron paramagnetic resonance (EPR) spectroscopy.Methods. 2016 Oct 15;109:31-43. doi: 10.1016/j.ymeth.2016.05.001. Epub 2016 May 6. Methods. 2016. PMID: 27163864 Review.
-
Medium-throughput ESR detection of superoxide production in undetached adherent cells using cyclic nitrone spin traps.Free Radic Res. 2015;49(9):1122-8. doi: 10.3109/10715762.2015.1045504. Epub 2015 Jun 5. Free Radic Res. 2015. PMID: 25968949
-
Detection of nitric oxide and superoxide radical anion by electron paramagnetic resonance spectroscopy from cells using spin traps.J Vis Exp. 2012 Aug 18;(66):e2810. doi: 10.3791/2810. J Vis Exp. 2012. PMID: 22929836 Free PMC article.
-
Imaging free radicals in organelles, cells, tissue, and in vivo with immuno-spin trapping.Redox Biol. 2016 Aug;8:422-9. doi: 10.1016/j.redox.2016.04.003. Epub 2016 Apr 22. Redox Biol. 2016. PMID: 27203617 Free PMC article. Review.
Cited by
-
Bioorthogonal Ligations and Cleavages in Chemical Biology.ChemistryOpen. 2020 Aug 14;9(8):835-853. doi: 10.1002/open.202000128. eCollection 2020 Aug. ChemistryOpen. 2020. PMID: 32817809 Free PMC article. Review.
References
-
- Halliwell B, Trends in Pharmacol. Sci, 2011, 32, 125. - PubMed
- Villamena FA, Molecular Basis of Oxidative Stress, John Wiley & Sons, Inc., 2013.
- Halliwell B and Gutteridge JM, Free Radicals in Biology and Medicine Oxford University Press, Oxford, UK, 4th edn., 2007.
-
- Hamer J and Macaluso A, Chem. Rev, 1964, 64, 473.
- Grigor’ev IA, in Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis John Wiley & Sons, Inc., 2nd edn., 2008, pp. 129–434.
- Yang J, Synlett, 2012, 23, 2293.
-
- Janzen EG, Acc. Chem. Res, 1971, 4, 31.
- Villamena FA and Zweier JL, Antioxid. Redox Signal, 2004, 6, 619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

