Glucocorticoid receptor inhibits Müller glial galectin-1 expression via DUSP1-dependent and -independent deactivation of AP-1 signalling
- PMID: 31328390
- PMCID: PMC6787449
- DOI: 10.1111/jcmm.14559
Glucocorticoid receptor inhibits Müller glial galectin-1 expression via DUSP1-dependent and -independent deactivation of AP-1 signalling
Abstract
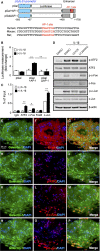
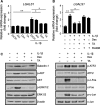
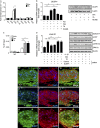
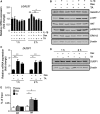
Galectin-1/LGALS1 is a hypoxia-induced angiogenic factor associated with diabetic retinopathy (DR). Recently, we elucidated a hypoxia-independent pathway to produce galectin-1 in Müller glial cells stimulated by interleukin (IL)-1β. Here we revealed glucocorticoid receptor (GR)-mediated inhibitory mechanisms for Müller glial galectin-1/LGALS1 expression. Activator protein (AP)-1 site in the LGALS1 enhancer region, to which activating transcription factor2, c-Fos and c-Jun bind, was shown to be essential for IL-1β-induced galectin-1/LGALS1 expression in Müller cells. Ligand (dexamethasone or triamcinolone acetonide)-activated GR induced dual specificity phosphatase (DUSP)1 expression via the glucocorticoid response element and attenuated IL-1β-induced galectin-1/LGALS1 expression by reducing phosphorylation of these AP-1 subunits following AKT and extracellular signal-regulated kinase (ERK)1/2 deactivation. Moreover, activated GR also caused DUSP1-independent down-regulation of IL-1β-induced LGALS1 expression via its binding to AP-1. Administration of glucocorticoids to mice attenuated diabetes-induced retinal galectin-1/Lgals1 expression together with AKT/AP-1 and ERK/AP-1 pathways. Supporting these in vitro and in vivo findings, immunofluorescence analyses showed co-localization of galectin-1 with GR and phosphorylated AP-1 in DUSP1-positive glial cells in fibrovascular tissues from patients with DR. Our present data demonstrated the inhibitory effects of glucocorticoids on glial galectin-1 expression via DUSP1-dependent and -independent deactivation of AP-1 signalling (transactivation and transrepression), highlighting therapeutic implications for DR.
Keywords: Müller glia; activator protein-1; diabetic retinopathy; dual specificity phosphatase 1; galectin-1; glucocorticoid receptor; interleukin-1β; transactivation; transrepression.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin‐1: a small protein with major functions. Glycobiology. 2006;16:137R‐157R. - PubMed
-
- Croci D, Cerliani J, Dalotto‐Moreno T, et al. Glycosylation‐dependent lectin‐receptor interactions preserve angiogenesis in anti‐VEGF refractory tumors. Cell. 2014;156:744‐758. - PubMed
-
- Yang N, Zhang W, He T, Xing Y. Silencing of galectin‐1 inhibits retinal neovascularization and ameliorates retinal hypoxia in a murine model of oxygen‐induced ischemic retinopathy. Exp Eye Res. 2017;159:1‐15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

