Discontinuation due to immune-related adverse events is a possible predictive factor for immune checkpoint inhibitors in patients with non-small cell lung cancer
- PMID: 31328416
- PMCID: PMC6718019
- DOI: 10.1111/1759-7714.13149
Discontinuation due to immune-related adverse events is a possible predictive factor for immune checkpoint inhibitors in patients with non-small cell lung cancer
Abstract
Background: Immune-related adverse events (irAEs) should be anticipated with treatment by immune checkpoint inhibitors (ICIs). Although the relationship between irAEs and efficacy of ICI has been reported, it has not yet been clarified whether the benefit from ICI outweighs the low frequency of proceeding to subsequent therapies after discontinuation due to irAEs.
Methods: The study comprised 61 patients with non-small cell lung cancer who underwent treatment with ICIs (nivolumab or pembrolizumab monotherapy) at the Saga University Medical School Hospital from December 2015 to January 2018. Therapeutic effect and progression-free survival (PFS) were compared between the irAEs discontinuation group (AEg) and the group with discontinuation due to all causes other than irAEs (Non-AEg).
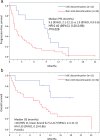
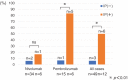
Results: A total of 30% patients(18/61) had therapy discontinued due to irAEs: 22.5% (9/40) with nivolumab and 42.9% (9/21) with pembrolizumab. The response rate was 50.0% in the AEg and 8.1% in the on-AEg (P = 0.001). The median PFS was significantly longer in the AEg (9.3 months; 95% CI 2.1-12.1) than in the non-AEg (1.9 months; 95% CI 0.9-3.6): HR 0.45 (95%CI 0.20-0.89; log-rank test P = 0.026). The prevalence of drug-induced interstitial lung disease (ILD) was 6.1% (3/49) in cases without interstitial pneumonia (IP) as the underlying disease, whereas it was 50% (6/12) in cases with IP (P = 0.001).
Conclusion: Discontinuation of treatment with ICIs due to irAEs predict a good response to ICIs and favorable outcome since their anti-cancer effects continue even after discontinuation. However, the presence of IP as the underlying disease increases the risk of drug-related ILD onset.
Keywords: Immune checkpoint inhibitor; immune-related adverse event; interstitial lung disease; non-small cell lung cancer; predictive factor.
© 2019 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures

References
-
- Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. - PubMed
-
- Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. - PubMed
-
- Schvartsman G, Peng SA, Bis G et al Response rates to single‐agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non‐small cell lung cancer. Lung Cancer 2017; 112: 90–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

