Nitric oxide maintains endothelial redox homeostasis through PKM2 inhibition
- PMID: 31328803
- PMCID: PMC6717893
- DOI: 10.15252/embj.2018100938
Nitric oxide maintains endothelial redox homeostasis through PKM2 inhibition
Abstract
Decreased nitric oxide (NO) bioavailability and oxidative stress are hallmarks of endothelial dysfunction and cardiovascular diseases. Although numerous proteins are S-nitrosated, whether and how changes in protein S-nitrosation influence endothelial function under pathophysiological conditions remains unknown. We report that active endothelial NO synthase (eNOS) interacts with and S-nitrosates pyruvate kinase M2 (PKM2), which reduces PKM2 activity. PKM2 inhibition increases substrate flux through the pentose phosphate pathway to generate reducing equivalents (NADPH and GSH) and protect against oxidative stress. In mice, the Tyr656 to Phe mutation renders eNOS insensitive to inactivation by oxidative stress and prevents the decrease in PKM2 S-nitrosation and reducing equivalents, thereby delaying cardiovascular disease development. These findings highlight a novel mechanism linking NO bioavailability to antioxidant responses in endothelial cells through S-nitrosation and inhibition of PKM2.
Keywords: Pyruvate kinase M2; S-nitrosation; cardiovascular disease; eNOS tyrosine phosphorylation; endothelial dysfunction.
© 2019 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

- A
Volcano plot highlighting proteins significantly enriched (open circles, FDR < 0.05) in eNOS‐FLAG immunoprecipitates from growth factor‐stimulated human endothelial cells; n = 3 independent cell batches.
- B
Nitrite in the cell supernatant of HEK293 cells expressing wild‐type (WT), Y657F (YF), or Y657D (YD) eNOS and treated with solvent (Sol) or ionomycin (Io, 1 μmol/l, 15 min); n = 6 independent experiments (2‐way ANOVA and Bonferroni).
- C
The FMN/FAD ratio measured in eNOS immunoprecipitates from cells expressing WT, YF, or YD eNOS; n = 6 independent experiments (1‐way ANOVA and Newman–Keuls).
- D
The consequence of mutating Y657 on the growth factor‐induced formation of eNOS complexes with Hsp90 and calmodulin (CaM); n = 6–19 independent cell batches (1‐way ANOVA and Newman–Keuls).
- E, F
Effect of mutating Y657 on the formation of complexes between eNOS, Hsp90, and PKM2 in growth factor‐stimulated human endothelial cells. Complex formation was assessed in eNOS‐FLAG immunoprecipitates (E), or PKM2 immunoprecipitates (F); n = 4–8 independent cell batches (1‐way ANOVA and Newman–Keuls).

The consequence of mutating Y657 on the growth factor‐induced formation of eNOS complexes with Hsp90, CaM, and PKM2 in human endothelial cells as summarized in Fig 1. Human endothelial cells expressing GFP were included as control. Complex formation was assessed in eNOS‐FLAG immunoprecipitates.
Formation of complexes between PKM2 and Hsp90 in the absence or presence of eNOS in HEK293 cells. Similar results were obtained in three additional experiments.
Effect of mutating Ser633 to Asp (SD) on the formation of complexes between eNOS, Hsp90, and PKM2 in growth factor‐stimulated human endothelial cells. Complex formation was assessed in eNOS‐myc immunoprecipitates; n = 4 independent cell batches. Data are presented as mean ± SEM.

Pyruvate kinase (PK) activity in FLAG immunoprecipitates from human endothelial cells expressing FLAG‐tagged wild‐type (WT), Y657F (YF), or Y657D (YD) eNOS; n = 6 independent cell batches (1‐way ANOVA and Tukey).
PK activity in eNOS immunoprecipitates from HEK293 cells expressing WT, YF, YD, or S633D (SD) eNOS; n = 5–9 independent experiments (1‐way ANOVA and Bonferroni).
S‐Nitrosation of PKM2 in human endothelial cells expressing FLAG‐tagged WT or YF‐eNOS and treated with H2O2 (30 μmol/l) for 15 min; n = 8 independent cell batches (Unpaired Student's t‐test). Dithiothreitol (DTT) was included to demonstrate specificity of the SNO signal.
PKM2 S‐nitrosation in HEK293 cells expressing YF or YD eNOS and either FLAG‐tagged WT PKM2 or a C358S (CS) PKM2 mutant. SNO‐FLAG‐PKM2 was detected with an anti‐FLAG antibody after biotin switch technique; n = 4 independent experiments (2‐way ANOVA and Bonferroni). DTT treatment was included to demonstrate specificity of the SNO signal.
PK activity in eNOS immunoprecipitates from HEK293 cells co‐expressing YF or YD eNOS and either WT PKM2 or the CS PKM2 mutant; n = 6 independent experiments (2‐way ANOVA and Bonferroni).
PKM2 enzyme kinetics measured with increasing concentrations of phosphoenolpyruvate (PEP) in eNOS immunoprecipitates from HEK293 cells expressing YF or YD eNOS. The data were fit with the Michaelis–Menten equation to determine V max and K m; n = 4 independent experiments (2‐way ANOVA and Bonferroni).
Quantification of pentose phosphate pathway (PPP) intermediates; gluconate‐6‐P (G6P), ribulose‐5‐P (Rl5P), ribose‐5‐P (R5P), sedoheptulose‐7‐P (S7P), fructose‐6‐P (F6P) and erythrose‐4‐P (E4P) in human endothelial cells expressing YF or YD eNOS; n = 6–8 independent cell batches (Unpaired Student's t‐test).
Link between the pentose phosphate pathway, generation of NADPH, and reduction of GSSG to GSH.
NADPH/NADP+ and GSH/GSSG ratios in human endothelial cells expressing YF or YD eNOS; n = 6–8 independent cell batches (Unpaired Student's t‐test).
NADPH/NADP+ and GSH/GSSG ratios in human endothelial cells treated with solvent (Sol) or the PKM2 inhibitor shikonin (SKN, 1 μmol/l) for 45 min; n = 4 independent cell batches (Unpaired Student's t‐test).

Schematic representation of the murine eNOS gene and of the targeting vector used to introduce the Y656F mutation.
Schematic representation of the Y656F eNOS knock‐in allele. The positive selection cassette (puromycin) flanked by F3 sites was deleted by crossing with a ubiquitously expressing FLP1 recombinase mouse strain.
The Y656F mutation generates a BsmI restriction site, allowing identification of the wild‐type, heterozygous, and knock‐in alleles by PCR and subsequent BsmI digestion.
Genotyping PCR using the primers described in the Materials and Methods section.

- A
Systolic blood pressure (SBP) in wild‐type (WT) and YF‐eNOS (YF) mice; n = 9 animals per group (Unpaired Student's t‐test).
- B, C
Phenylephrine (PE)‐induced contraction (B) and acetylcholine (Ach)‐induced relaxation (C) of endothelium‐intact aortic rings from WT and YF mice. Experiments were performed in the absence and presence of L‐NAME (LN, 300 μmol/l); n = 6–10 animals per group (2‐way ANOVA and Bonferroni).
- D
Acetylcholine (ACh)‐induced vasodilatation of the buffer‐perfused hindlimb in situ. Experiments were performed in the absence and presence of L‐NAME (LN, 300 μmol/l); n = 12–13 animals per group (2‐way ANOVA and Bonferroni).
- E
PKM2 S‐nitrosation in pulmonary endothelial cells from WT and YF mice; n = 5–6 independent cell batches (Unpaired Student's t‐test). Dithiothreitol (DTT) treatment was included to demonstrate specificity of the SNO signal. The blots show non‐adjacent bands cropped from the same membranes.
- F
PK activity in eNOS immunoprecipitates from WT and YF pulmonary endothelial cells; n = 7–10 independent cell batches (Unpaired Student's t‐test).
- G
PK activity in eNOS immunoprecipitates from hearts from WT and YF mice; n = 6 mice per group (Unpaired Student's t‐test).
- H
Representative images of eNOS‐PKM2 interaction (PLA foci, red) in mouse pulmonary endothelial cells; the Golgi apparatus and endosomes were stained with Golph4 (green), the plasma membrane was stained with CD144, and nuclei were highlighted with DAPI (gray). Only rare PLA foci were found in samples incubated with control mouse and rabbit IgGs, demonstrating the specificity of the reaction; n = 3 independent cell batches. Scale bars: 20 μm.
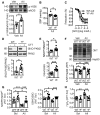
Phosphorylation of eNOS on Tyr656 in lung lysates from animals treated for 7 days; n = 6–7 mice per group (Unpaired Student's t‐test).
Systolic blood pressure (SBP) in WT and YF mice treated for 28 days; n = 10 mice per group (Unpaired Student's t‐test).
Acetylcholine (ACh)‐induced relaxation of aortic rings from the same animals as in panel B; n = 8 mice per group (2‐way ANOVA and Bonferroni).
PKM2 S‐nitrosation in lungs from WT and YF mice administered AII for 28 days; n = 7–8 mice per group (Unpaired Student's t‐test). DTT treatment was included to demonstrate specificity of the SNO signal. The blots show non‐adjacent lanes from the same membranes.
NADPH/NADP+ and GSH/GSSG ratios in lungs from WT and YF mice treated with AII for 28 days; n = 8–9 mice per group (Unpaired Student's t‐test).
3‐Nitrotyrosine (3‐NT) levels in lungs from WT and YF mice treated with vehicle (Veh) or AII (AII) for 28 days; n = 7–9 mice per group (2‐way ANOVA and Bonferroni).
NADPH/NADP+ and GSH/GSSG ratios in pulmonary endothelial cells from WT and YF mice treated with AII for 30 min; n = 8 cell batches (2‐way ANOVA and Tukey).
H2O2 levels in pulmonary endothelial cells from WT and YF mice after treatment with AII (1 μmol/l) for 30 min; n = 8 cell batches (2‐way ANOVA and Tukey).

Proximity ligation assays showing S‐nitrosated PKM2 (yellow) in the RCA and LCA 2 days after ligation. Phalloidin staining (blue) was included to highlight the vessel wall; n = 4–5 mice per group (2‐way ANOVA and Bonferroni). Scale bars: 50 μm.
GSH (green) levels in the RCA and LCA 2 days after ligation. CD31 (red) was included to label endothelial cells. n = 4–5 mice per group (2‐way ANOVA and Bonferroni). Scale bars: 50 μm.
3‐nitrotyrosine (3NT, green) levels in the RCA and LCA 2 days after ligation. CD31 (red) was included to label endothelial cells. n = 5 mice per group (2‐way ANOVA and Bonferroni). Scale bars: 50 μm.
Acetylcholine (ACh)‐induced relaxation of the phenylephrine contracted LCA 7 days after ligation; n = 7–8 mice per group (2‐way ANOVA and Bonferroni).
Acetylcholine (ACh)‐induced relaxation of the phenylephrine contracted aorta 7 days after ligation; n = 7–8 mice per group (2‐way ANOVA and Bonferroni).
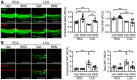
GSH (green) levels in the RCA and LCA 3 days after ligation. CD31 (red) was included to label endothelial cells. n = 5 mice per group (2‐way ANOVA and Bonferroni). Scale bars: 50 μm.
3‐Nitrotyrosine (3NT, green) levels in the RCA and LCA 3 days after ligation. CD31 (red) was included to label endothelial cells. n = 5 mice per group (2‐way ANOVA and Bonferroni). Scale bars: 50 μm.

- A
ICAM1 and VCAM1 (green) expression in the LCA 2 days after ligation. CD31 (red) was included to label endothelial cells, and nuclei are highlighted with DAPI (gray). Comparable images were obtained in four additional animals in each group. Scale bars: 50 μm.
- B–D
Percentage of neutrophils and monocytes infiltrated into the LCA 7 days after ligation and high‐cholesterol diet feeding; n = 9–10 mice per group (Unpaired Student's t‐test).
- E
Representative images and quantification of ICAM1 (green) and VCAM1 (red) staining in human endothelial cells pre‐treated with the PKM2 inhibitor shikonin (SKN, 1 μmol/l) or solvent (Sol) for 45 min and then stimulated with interleukin‐1β (IL‐1, 20 ng/ml) or solvent (CTL) for 90 min. Nuclei are highlighted with DAPI (gray). n = 6 independent cell batches (2‐way ANOVA and Bonferroni). Scale bars: 20 μm.
- F, G
Percentage of neutrophils and macrophages infiltrated into the LCA from ApoE−/− mice treated with Veh or SKN (1.2 mg/kg) 7 days after ligation and high‐cholesterol diet feeding; n = 4–6 mice per group (Unpaired Student's t‐test).

- A
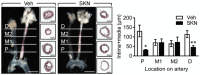
Plaque burden in ligated carotid arteries from ApoE−/− (−/−) and ApoEYF (YF) mice fed a high‐cholesterol diet for 21 days; scale bars: 2 mm. Intimal and medial thickness was determined at four specific locations (proximal, middle 1, middle 2, and distal) in cryo‐sectioned arteries stained with Oil Red O; scale bars: 200 μm. n = 7 mice per group (Unpaired Student's t‐test).
- B, C
Oil Red O staining of plaques in aortae from mice fed a Western diet for four (B) or 6 months (C); scale bars: 3 mm. n = 6 (B) or n = 12 (C) mice per group (Unpaired Student's t‐test).
References
-
- Boo YC, Sorescu GP, Bauer PM, Fulton D, Kemp BE, Harrison DG, Sessa WC, Jo H (2003) Endothelial NO synthase phosphorylated at SER635 produces NO without requiring intracellular calcium increase. Free Radic Biol Med 35: 729–741 - PubMed
-
- Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X (2011) Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase‐M2. Oncogene 30: 4297–4306 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

