Clinical Trial in a Dish: Personalized Stem Cell-Derived Cardiomyocyte Assay Compared With Clinical Trial Results for Two QT-Prolonging Drugs
- PMID: 31328865
- PMCID: PMC6853144
- DOI: 10.1111/cts.12674
Clinical Trial in a Dish: Personalized Stem Cell-Derived Cardiomyocyte Assay Compared With Clinical Trial Results for Two QT-Prolonging Drugs
Abstract
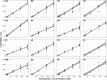
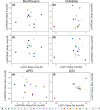
Induced pluripotent stem cells (iPSCs) have shown promise in investigating donor-specific phenotypes and pathologies. The iPSC-derived cardiomyocytes (iPSC-CMs) could potentially be utilized in personalized cardiotoxicity studies, assessing individual proarrhythmic risk. However, it is unclear how closely iPSC-CMs derived from healthy subjects can recapitulate a range of responses to drugs. It is well known that QT-prolonging drugs induce subject-specific clinical response and that all healthy subjects do not necessarily develop arrhythmias or exhibit similar amounts of QT prolongation. We previously reported this variability in a study of four human ether-a-go-go-related gene (hERG) potassium channel-blocking drugs in which each subject underwent intensive pharmacokinetic and pharmacodynamic sampling such that subjects had 15 time-matched plasma drug concentration and electrocardiogram measurements throughout 24 hours after dosing in a phase I clinical research unit. In this study, iPSC-CMs were generated from those subjects. Their drug-concentration-dependent QT prolongation response from the clinic was compared with in vitro drug-concentration-dependent action potential duration (APD) prolongation response to the same two hERG-blocking drugs, dofetilide and moxifloxacin. Comparative results showed no significant correlation between the subject-specific APD response slopes and clinical QT response slopes to either moxifloxacin (P = 0.75) or dofetilide (P = 0.69). Similarly, no significant correlation was found between baseline QT and baseline APD measurements (P = 0.93). This result advances our current understanding of subject-specific iPSC-CMs and facilitates discussion into factors obscuring correlation and considerations for future studies of subject-specific phenotypes in iPSC-CMs.
© 2019 The Authors. Clinical and Translational Science published by Wiley Periodicals Inc. on behalf of the American Society of Clinical Pharmacology & Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work. J.C.W. is a co‐founder of Khloris Biosciences but has no competing interests, as the work presented was performed independently.
Figures




References
-
- Takahashi, K. & Yamanaka, S. Induced pluripotent stem cells in medicine and biology. Development 140, 2457–2461 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

