Fatty acid and retinol-binding protein: A novel antigen for immunodiagnosis of human strongyloidiasis
- PMID: 31329601
- PMCID: PMC6645452
- DOI: 10.1371/journal.pone.0218895
Fatty acid and retinol-binding protein: A novel antigen for immunodiagnosis of human strongyloidiasis
Abstract
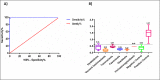
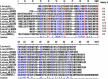
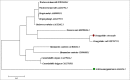
The tenacious human parasitic helminth Strongyloides stercoralis is a significant health problem worldwide. The current lack of a definitive diagnostic laboratory test to rule out this infection necessitates designing more specific diagnostic methods. Fatty acid and retinol-binding protein (FAR) plays a crucial role in the development and reproduction of nematodes. We generated a recombinant form of this protein and determined its applicability for immunodiagnosis of S. stercoralis. The L3 form of S. stercoralis was harvested and used for RNA extraction and cDNA synthesis. The coding sequence of S. stercoralis FAR (SsFAR) was cloned into pET28a(+) vector, expressed in E. coli BL21 and purified. ELISA and immunoblotting were employed to determine the specificity and sensitivity of rSsFAR using a set of defined sera. In addition, we analyzed the phylogenetic relationship of SsFAR with different FAR sequences from other nematodes. The cloned SsFAR had an open reading frame of 447 bp encoding 147 amino acids, with a deduced molecular mass of 19 kD. The SsFAR amino acid sequence was 93% identical to FAR of S. ratti. For differential immunodiagnosis of strongyloidiasis, rSsFAR exhibited 100% sensitivity and 97% specificity. However, cross-reactivity with FAR proteins of other parasites, namely Toxocara canis and Echinococcus granulosus, was noted. Our results provide a novel approach for immunodiagnosis of S. stercoralis infections using rSsFAR with reliable sensitivity and specificity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Kia E, Mahmoudi M, Zahabiun F, Meamar A. An evaluation on the efficacy of agar plate culture for detection of Strongyloides stercoralis. Iran J Parasitol. 2007;2(1):29–34.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

