Preservation of cellular nano-architecture by the process of chemical fixation for nanopathology
- PMID: 31329606
- PMCID: PMC6645510
- DOI: 10.1371/journal.pone.0219006
Preservation of cellular nano-architecture by the process of chemical fixation for nanopathology
Abstract
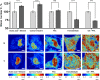
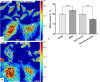
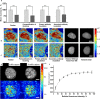
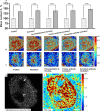
Transformation in chromatin organization is one of the most universal markers of carcinogenesis. Microscale chromatin alterations have been a staple of histopathological diagnosis of neoplasia, and nanoscale alterations have emerged as a promising marker for cancer prognostication and the detection of predysplastic changes. While numerous methods have been developed to detect these alterations, most methods for sample preparation remain largely validated via conventional microscopy and have not been examined with nanoscale sensitive imaging techniques. For these nanoscale sensitive techniques to become standard of care screening tools, new histological protocols must be developed that preserve nanoscale information. Partial Wave Spectroscopic (PWS) microscopy has recently emerged as a novel imaging technique sensitive to length scales ranging between 20 and 200 nanometers. As a label-free, high-throughput, and non-invasive imaging technique, PWS microscopy is an ideal tool to quantify structural information during sample preparation. Therefore, in this work we applied PWS microscopy to systematically evaluate the effects of cytological preparation on the nanoscales changes of chromatin using two live cell models: a drug-based model of Hela cells differentially treated with daunorubicin and a cell line comparison model of two cells lines with inherently distinct chromatin organizations. Notably, we show that existing cytological preparation can be modified in order to maintain clinically relevant nanoscopic differences, paving the way for the emerging field of nanopathology.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: Drs. Backman and Subramanian are cofounders/shareholders of Nanocytomics LLC. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures




References
-
- Creech MK, Wang J, Nan X, Gibbs SL. Superresolution Imaging of Clinical Formalin Fixed Paraffin Embedded Breast Cancer with Single Molecule Localization Microscopy. Sci Rep. 2017;7:40766 10.1038/srep40766 https://www.nature.com/articles/srep40766#supplementary-information. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

