Environmental degradation amplifies species' responses to temperature variation in a trophic interaction
- PMID: 31330040
- PMCID: PMC6899768
- DOI: 10.1111/1365-2656.13069
Environmental degradation amplifies species' responses to temperature variation in a trophic interaction
Abstract
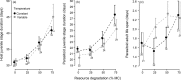
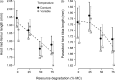
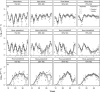
Land-use and climate change are two of the primary drivers of the current biodiversity crisis. However, we lack understanding of how single-species and multispecies associations are affected by interactions between multiple environmental stressors. We address this gap by examining how environmental degradation interacts with daily stochastic temperature variation to affect individual life history and population dynamics in a host-parasitoid trophic interaction, using the Indian meal moth, Plodia interpunctella, and its parasitoid wasp Venturia canescens. We carried out a single-generation individual life-history experiment and a multigeneration microcosm experiment during which individuals and microcosms were maintained at a mean temperature of 26°C that was either kept constant or varied stochastically, at four levels of host resource degradation, in the presence or absence of parasitoids. At the individual level, resource degradation increased juvenile development time and decreased adult body size in both species. Parasitoids were more sensitive to temperature variation than their hosts, with a shorter juvenile stage duration than in constant temperatures and a longer adult life span in moderately degraded environments. Resource degradation also altered the host's response to temperature variation, leading to a longer juvenile development time at high resource degradation. At the population level, moderate resource degradation amplified the effects of temperature variation on host and parasitoid populations compared with no or high resource degradation and parasitoid overall abundance was lower in fluctuating temperatures. Top-down regulation by the parasitoid and bottom-up regulation driven by resource degradation contributed to more than 50% of host and parasitoid population responses to temperature variation. Our results demonstrate that environmental degradation can strongly affect how species in a trophic interaction respond to short-term temperature fluctuations through direct and indirect trait-mediated effects. These effects are driven by species differences in sensitivity to environmental conditions and modulate top-down (parasitism) and bottom-up (resource) regulation. This study highlights the need to account for differences in the sensitivity of species' traits to environmental stressors to understand how interacting species will respond to simultaneous anthropogenic changes.
Keywords: climate change; environmental variation; habitat modification; host-parasitoid; life-history trajectories; phenological mismatch; population cycles; population dynamics.
© 2019 The Authors. Journal of Animal Ecology published by John Wiley & Sons Ltd on behalf of British Ecological Society.
Figures


References
-
- Begon, M. , Sait, S. M. , & Thompson, D. J. (1995) Persistence of a parasitoid‐host system – Refuges and generation cycles. Proceedings of the Royal Society B‐Biological Sciences, 260, 131–137.
-
- Begon, M. , Sait, S. M. , & Thompson, D. J. (1996). Predator‐prey cycles with period shifts between two‐ and three‐species systems. Nature, 381, 311–315. 10.1038/381311a0 - DOI
-
- Belarde, T. A. , & Railsback, S. F. (2016). New predictions from old theory: Emergent effects of multiple stressors in a model of piscivorous fish. Ecological Modelling, 326, 54–62. 10.1016/j.ecolmodel.2015.07.012 - DOI
-
- Benrey, B. , & Denno, R. F. (1997). The slow‐growth‐high‐mortality hypothesis: A test using the cabbage butterfly. Ecology, 78, 987–999.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

