Pharmacological characterization of the seven human NOX isoforms and their inhibitors
- PMID: 31330481
- PMCID: PMC6658998
- DOI: 10.1016/j.redox.2019.101272
Pharmacological characterization of the seven human NOX isoforms and their inhibitors
Abstract
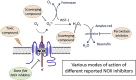
Background: NADPH oxidases (NOX) are a family of flavoenzymes that catalyze the formation of superoxide anion radical (O2•-) and/or hydrogen peroxide (H2O2). As major oxidant generators, NOX are associated with oxidative damage in numerous diseases and represent promising drug targets for several pathologies. Various small molecule NOX inhibitors are used in the literature, but their pharmacological characterization is often incomplete in terms of potency, specificity and mode of action.
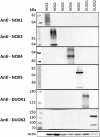
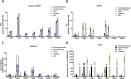
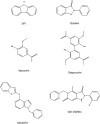
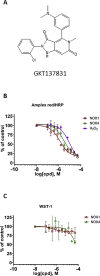
Experimental approach: We used cell lines expressing high levels of human NOX isoforms (NOX1-5, DUOX1 and 2) to detect NOX-derived O2•- or H2O2 using a variety of specific probes. NOX inhibitory activity of diphenylene iodonium (DPI), apocynin, diapocynin, ebselen, GKT136901 and VAS2870 was tested on NOX isoforms in cellular and membrane assays. Additional assays were used to identify potential off target effects, such as antioxidant activity, interference with assays or acute cytotoxicity.
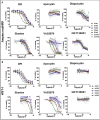
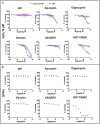
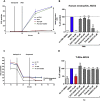
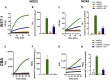
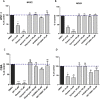
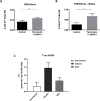
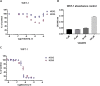
Key results: Cells expressing active NOX isoforms formed O2•-, except for DUOX1 and 2, and in all cases activation of NOX isoforms was associated with the detection of extracellular H2O2. Among all molecules tested, DPI elicited dose-dependent inhibition of all isoforms in all assays, however all other molecules tested displayed interesting pharmacological characteristics, but did not meet criteria for bona fide NOX inhibitors.
Conclusion: Our findings indicate that experimental results obtained with widely used NOX inhibitors must be carefully interpreted and highlight the challenge of developing reliable pharmacological inhibitors of these key molecular targets.
Keywords: NADPH oxidase; NOX; Reactive oxygen species; Small molecule inhibitors.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87(1):245–313. - PubMed
-
- van der Weyden L., Arends M.J., Campbell A.D., Bald T., Wardle-Jones H., Griggs N., Velasco-Herrera M.D., Tuting T., Sansom O.J., Karp N.A., Clare S., Gleeson D., Ryder E., Galli A., Tuck E., Cambridge E.L., Voet T., Macaulay I.C., Wong K., Sanger Mouse Genetics P., Spiegel S., Speak A.O., Adams D.J. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature. 2017;541(7636):233–236. - PMC - PubMed
-
- Casas A.I., Geuss E., Kleikers P.W.M., Mencl S., Herrmann A.M., Buendia I., Egea J., Meuth S.G., Lopez M.G., Kleinschnitz C., Schmidt H. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl. Acad. Sci. U. S. A. 2017;114(46):12315–12320. - PMC - PubMed
-
- Di Marco E., Gray S.P., Kennedy K., Szyndralewiez C., Lyle A.N., Lassegue B., Griendling K.K., Cooper M.E., Schmidt H., Jandeleit-Dahm K.A.M. NOX4-derived reactive oxygen species limit fibrosis and inhibit proliferation of vascular smooth muscle cells in diabetic atherosclerosis. Free Radic. Biol. Med. 2016;97:556–567. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

