Optimization of the Electro-Peroxone Process for Micropollutant Abatement Using Chemical Kinetic Approaches
- PMID: 31330777
- PMCID: PMC6680746
- DOI: 10.3390/molecules24142638
Optimization of the Electro-Peroxone Process for Micropollutant Abatement Using Chemical Kinetic Approaches
Abstract
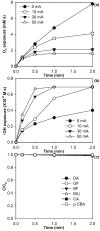
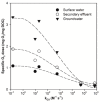
The electro-peroxone (E-peroxone) process is an emerging electrocatalytic ozonation process that is enabled by in situ producing hydrogen peroxide (H2O2) from cathodic oxygen reduction during ozonation. The in situ-generated H2O2 can then promote ozone (O3) transformation to hydroxyl radicals (•OH), and thus enhance the abatement of ozone-refractory pollutants compared to conventional ozonation. In this study, a chemical kinetic model was employed to simulate micropollutant abatement during the E-peroxone treatment of various water matrices (surface water, secondary wastewater effluent, and groundwater). Results show that by following the O3 and •OH exposures during the E-peroxone process, the abatement kinetics of a variety of model micropollutants could be well predicted using the model. In addition, the effect of specific ozone doses on micropollutant abatement efficiencies could be quantitatively evaluated using the model. Therefore, the chemical kinetic model can be used to reveal important information for the design and optimization of the treatment time and ozone doses of the E-peroxone process for cost-effective micropollutant abatement in water and wastewater treatment.
Keywords: electro-peroxone; electrocatalytic ozonation; model; ozone; pharmaceutical; water treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Guo W.Q., Wu Q.L., Zhou X.J., Cao H.O., Du J.S., Yin R.L., Ren N.Q. Enhanced amoxicillin treatment using the electro-peroxone process: Key factors and degradation mechanism. Rsc Adv. 2015;5:52695–52702. doi: 10.1039/C5RA07951A. - DOI
-
- Nawrocki J. Catalytic ozonation in water: Controversies and questions. Discussion paper. Appl. Catal. B Environ. 2013;142:465–471. doi: 10.1016/j.apcatb.2013.05.061. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

